|
Cell Biology: Osteoclasts and Related
Cytokines
P-111
EFFECTS OF REPEATED INGESTION OF CALCIUM-FORTIFIED SPRING WATER ON SERUM
C-TERMINAL CROSS-LINKING TELOPEPTIDE OF TYPE I COLLAGEN (CTX)
J. A. Guillemant1, C. Accarie2, V. de la
Guéronničre3, S. E. Guillemant1,2*
1Faculté de Médecine Pitié-Salpźtričre, Paris, France
2EPHE, Nutrition Hydrominérale, Paris, France
3Pōle Expertise Eau, Bourg-la-Reine, France
In a previous study (Nutrition Research, 2002) we showed
that repeated ingestion of calcium-rich water could significantly increase the serum
concentration of ionized calcium and suppress serum CTX demonstrating that calcium-rich
mineral water can effectively suppress bone resorption. Nevertheless, the availability of
natural calcium- rich mineral water is limited and the possibility to fortify natural
spring water with calcium salt is to be considered. Fifteen young adult males (21-26 y)
ingested at three times (08.00, 11.00 and 14.00) 660 ml of either calcium-fortified spring
water (300 mg/L of elemental calcium) or calcium-poor (<10 mg/L) mineral water. Blood
was collected before 08.00 and at 11.00, 14.00 and 17.00 for measurement of serum ionized
calcium and CTX. After every intake of calcium-fortified water, serum ionized calcium
increased (from 1.25 to 1.31 mmol/L) while CTX decreased (by 60%) gradually. Since
ingestion of calcium-poor mineral water induced a modest but significant decrease in CTX
we compared the two sets of trials with repeated- measures two-factor analysis of variance
with interaction. Ingestion of calcium- fortified water resulted in a significant decrease
in serum CTX (time, P<0.0001; treatment, P<0.0001; time-by-treatment, P<0.0001)
demonstrating that usually drinking calcium-fortified water could efficiently suppress
bone resorption throughout the day.
[Programme]
P-112
EFFECT OF RANKL ON THE FORMATION OF OSTEOCLASTS GENERATED FROM PERIPHERAL
BLOOD MONONUCLEAR CELLS: CORRELATION BETWEEN AREA OF RESORPTION AND RELEASE OF BETA
C-TERMINAL TELOPEPTIDES
B. Y. Y. Chan1*, K. A. Buckley2, J. Dutton1,
J. A. Gallagher2, W. D. Fraser1
1Department of Clinical Chemistry, Royal Liverpool University
Hospital, The University of Liverpool, Liverpool, L69 3GA, UK
2Human Bone Cell Research Group, Department of Human Anatomy
and Cell Biology, The University of Liverpool, Liverpool, L69 3GE, UK
BACKGROUND: The receptor activator for nuclear
factor-kappaB ligand (RANKL) is a critical factor for osteoclast (OC) differentiation,
survival and maturation. The development of techniques that generate OCs in vitro
from peripheral blood mononuclear cells (PBMCs) have provided methods of producing
unlimited supplies of OCs which were previously difficult to obtain. The current method to
quantify the resorptive activity of OCs by point counting using reflected light microscopy
is time consuming and can lead to inaccuracies. The bone specific resorption marker beta
c-terminal telopeptide (beta-CTx) from degradation of type I collagen may provide an
alternative measurement for OC resorption. This study evaluated the effect of RANKL in
stimulating OC activity, and correlated the release of beta-CTx from dentine wafers with
the area of resorption produced by OCs. METHOD: Venous blood from patients with Paget's
disease of bone and healthy volunteers was collected and PBMCs were isolated. PBMCs from
Paget's subjects were cultured in medium containing either 10% FCS, or 1% or 10% human
serum derived from the same individual, 30 nano-grams per millilitre RANKL, and 25 nano-
grams per millilitre macrophage-colony-stimulating factor (M-CSF). PBMCs from normal
subjects were cultured in the presence of 30, or 60 nano-grams per millilitre RANKL, 25
nano-grams per millilitre M-CSF, and 10% FCS. Cell culture medium was harvested at
specific time points (7, 14, and 21 days) during a 3-week incubation for measurement of
beta-CTx by electrochemiluminescence immunoassay (ROCHE, Lewes UK). End-point area of
resorption was evaluated by point counting using reflected light microscopy. RESULTS:
Incubation of Pagetic PBMCs with 10% Pagetic serum or 10% FCS resulted in a significantly
higher release of beta-CTx and a greater area of resorption than from cells cultured in 1%
Pagetic serum. Beta-CTx was significantly correlated with the area of resorption (r=0.92,
n=62). Beta-CTx release from cultures of 'healthy' PBMCs was also significantly correlated
with the increasing area of resorption produced from OC formed in the presence of
increasing concentrations of RANKL (r=0.97, n=13). CONCLUSION: Use of beta-CTx as a marker
for bone resorption activity could replace the laborious method of point counting
resorption lacunae produced by recombinant RANKL-generated OCs.
[Programme]
P-113
SIMULATED RATES OF RESORPTION DURING CANCELLOUS BONE REMODELING USING
MICHAELIS-MENTEN (M-M) EQUATIONS AND THE INTERACTIVE EFFECTS OF RANKL, RANK AND OPG ON
OSTEOCLAST ACTIVITY
M. J. Martin*, J. C. Buckland-Wright
GKT School of Biomedical Sciences, King's College, London, UK
The development of pharmaceutical treatments for bone
disease can be enhanced by predicting the effects of disturbance on the process of bone
resorption during cancellous bone remodeling. We present a mathematical model to simulate
the rates of cellular activity during the resorption phase of remodeling in healthy bone,
controlled by interactions between matrix, marrow and vascular tissue.
Rates of cellular activity during resorption within a
representative volume of bone are calculated on a daily time step by the M-M kinetic
equations that describe enzyme and cellular activity. Osteoclastic activity, AOcl,
is simulated by the interaction between RANKL, RANK and the competitive inhibition of OPG,
evident as the observed dependency of osteoclast survival and function on the ratio of
RANKL to OPG. The ratio of RANKL to OPG is representd as the effective concentration of
RANKL, [RANKLEff], and the required presence of stromal cell production of M-
CSF is represented as factor FM-CSF, which is equal to 1 in normal, healthy
bone.
AOcl = [(VOclmax * [RANKLEff])
/ ([RANKLEff] + KOclM)] * FM-CSF
where VOclmax is the maximum rate of
osteoclastic activity, and KOclMis the M-M constant.
The release of factors such as TGFbeta1 from the matrix
are tracked, and the decrease in osteoclastic resorption with time is simulated by the
negative feedback effect of released TGFbeta1 on OPG production by marrow stromal cells.
Apoptosis and cessation of osteoclastic resorption is assumed to occur at a threshold
level of TGFbeta1 released from the matrix. The rate of collagen fibril removal during the
second phase of resorption is simulated by the activity of mono-nucleated lining cells,
using the M-M kinetic equation.
The model is parameterised using published data (for
example, Eriksen et al., 1984; Parfitt et al., 1996; Fuller et al.,
1998).
This is the first mathematical model that uses the M-M
equations adapted for cellular activity to simulate the rate of resorption during the
remodeling of cancellous bone.
[Programme]
P-114
TARTRATE-RESISTANT ACID PHOSPHATASE FUNCTIONS AS A PROTEIN TYROSINE
PHOSPHATASE IN MOUSE LUNG TISSUE
P. Muhonen*, K. Kaarlonen, H. Ylipahkala, S. L. Alatalo, H. K.
Väänänen, J. M. Halleen
Institute of Biomedicine, Department of Anatomy, University of Turku,
Finland
Tartrate-resistant acid phosphatase (TRACP) is an enzyme
with unknown biological function. It is expressed primarily in bone-resorbing osteoclasts
and activated macrophages. TRACP knock-out mice develop mild osteopetrosis and show
reduced clearance of the pathogen S. Aureus, suggesting an important role for the enzyme
in bone resorption and immune defense system. We have used TRACP over- expressing (TRACP+)
mice to identify potential biological substrates for the phosphatase activity of the
enzyme. We performed Western-analysis to lung homogenates from wild-type (WT) and TRACP+
mice using phosphotyrosine and phosphoserine antibodies and quantitated band intensities
with image analysis. The specific activity of TRACP was significantly higher in lung
homogenates from TRACP+ mice compared with WT mice. Lung homogenates from TRACP+ mice
contained significantly lower amount of phosphotyrosine, but the amount of phosphoserine
was not different from WT mice. Two bands sized 120 kD and 180 kD were identified that
contained 90% less phosphotyrosine in lung homogenates from TRACP+ mice. The band
intensities showed a significant negative correlation with the specific activity of TRACP
in lung homogenates from TRACP+ mice. These results suggest that TRACP functions as a
protein tyrosine phosphatase, but not as a serine phosphatase, and that it may have at
least two different natural substrates in lung tissue.
[Programme]
P-115
CHARACTERIZATION OF CIRCULATING HUMAN OSTEOCLAST PRECURSORS
T. A. Hentunen*, H. Michael, H. K. Väänänen
Department of Anatomy, Institute of Biomedicine, University of Turku,
Turku, Finland
Osteoclasts are multinucleated bone-resorbing cells. They
are differentiated from hematopoietic precursors, including cells in the peripheral blood.
The phenotype of osteoclast precursor is still unknown. First, we developed a method to
induce high numbers of resorbing osteoclasts from the total peripheral blood mononuclear
cell (PBMC) fraction using a cocktail of growth factors: RANKL, M-CSF, TNF-alfa and
dexamethasone. PBMC were enriched using Ficoll isolation method. From one million plated
PBMC/bone slice formed between 100-500 tartrate-resistant acid phosphatase (TRACP)
-positive multinucleated cells (MNC) with 5 nuclei/cell on an average in a 14-day culture.
Osteoclast formation capacity was thus approximately 0.15 %. It appeared that lymphocytes
present in the culture interfered osteoclast formation causing variation and inhibition in
osteoclast maturation. Next, we purified monocytes up to purity of 99 % using
immunomagnetic cell separation system (MACS microbeads, Miltenyi, Germany). CD14+ cells as
plated at the cell density of 0.1 million cells/bone slice generated approximately 550
TRACP+ MNC with 5 nuclei/cell. Hence, the osteoclast formation capacity was between 2.75
%. Purified CD14+ cells had 15-20 fold higher osteoclast formation capacity compared to
whole PBMC fraction suggesting that osteoclast precursors reside in monocyte population.
CD11b+ cells formed approximately 180 TRACP+ MNC. This was 30 % of the number obtained
with CD14+ cells. CD61+ cells were also able to differentiate into resorbing
TRACP-positive MNC. Their osteoclast formation capacity was slightly lower than the one of
CD11b+ cells. CD169+ cells, however, did not form any osteoclastic cells. This was an
interesting observation suggesting that in the circulation there may be different monocyte
subpopulations, which are already committed to osteoclast, macrophage and dendritic cell
lineages. TRACP+ MNC generated in the culture expressed vacuolar proton ATPase and formed
typical actin rings on bone slices. Their resorption activity was high, the resorption
area being 50- 80 % of the bone slice area. In addition, calcitonin was found to inhibit
the resorption activity of osteoclast-like cells formed in the culture. To summarize,
circulating human osteoclast precursors are positive for CD14, CD11b and CD61 and negative
for CD15 and CD169 and they can be induced to differentiate into osteoclasts with high
resorption activity.
[Programme]
P-116
LOCALIZATION OF TRACP IN RESORBING OSTECLASTS
J. Vääräniemi*, J. Halleen, H. K. Väänänen
Department of Anatomy, Institute of Biomedicine, University of Turku,
Turku, Finland
Osteoclasts are multinucleated monocyte-macrophage
derivatives responsible for bone resorption. They dissolve bone mineral by secretion of
protons and degrade collagen matrix by secretion of proteolytic enzymes that are secreted
through the ruffled border (RB) membrane. RB faces the bone matrix and has late
endosomal/lysosomal characteristics but it is compositionally different from lysosomes.
Resorption products are endocytosed inside the cell through RB and secreted to the
extracellular space via a functional secretory domain in the basolateral
membrane. This route is defined as transcytotic pathway.
Osteoclasts also produce high amounts of reactive oxygen species (ROS) during resorption
without known function. In this study we used confocal microscopy to characterize the
precence and localization of tartarate resistant acid phosphatase (TRACP) in resorbing and
non- resorbing rat osteoclasts cultured on bovine bone. TRACP is a specific marker for
osteoclasts in bone and serum TRACP is a potential marker of bone resorption. Biological
function and subcellular localization of TRACP remains unclear although TRACP may have
functions as a protein tyrosine phosphatase and in iron metabolism. We studied the
colocalization of TRACP vesicles and secreted or endocytoted vesicles found in different
intravesicular pathways. Our results show that TRACP is present in both resorbing and
non-resobing osteoclasts and it is localized perinuclearly. Many TRACP-containing vesicles
are big compared to other vesicles. In resorbing osteoclasts some but not all TRACP
containing vesicles are acidic as indicated by DAMP that is concentrated in acidic
organelles. TRACP colocalizes with bone collagen degrading enzyme cathepsin K in the
cytoplasm and near basolateral membrane of resorbing osteoclast. TRACP also colocalizes
with fluorescently labelled bone in the cytoplasm but not in the bone surface level. Some
colocalization can be seen with transferrin, Rab3a and Rab9 proteins. TRACP does not
colocalize or colocalizes very little with late endosomal or lysosomal markers like Rab7
protein or LDL-containing lysosomes.
[Programme]
P-117
FOCUS ON BONE REMODELING IN CELIAC DISEASE
D. Fortunati1*, A. Taranta2, M. Longo1,
N. Rucci2, S. Migliaccio2,3, M. L. Bianchi4, M. T.
Bardella5, S. Saraifoger4, A. Teti2
1Dept of Histology and Medical Embryology, University of Rome
'La Sapienza', Rome, Italy
2Dept of Experimental Medicine, University of L'Aquila,
L'Aquila, Italy
3Dept of Medical Physiopathology, University of Rome 'La
Sapienza', Rome, Italy
4Bone Metabolic Unit, Istituto Auxologico Italiano-IRCCS,
Milan, Italy
5Cattedra di Gastroenterologia-IRCCS Ospedale Maggiore, Milan,
Italy
Gluten intolerance causes celiac disease. This
inflammatory disorder of the small bowel is often associated with low bone mass density
(BMD). To assess whether any factors not related to intestinal malabsorption were involved
in altered bone turnover, human peripheral blood monocytes were challenged for three weeks
with sera from 21 healthy donors, 17 just-diagnosed, yet untreated celiac patients, and 25
celiac patients on gluten-free diet for at least two years, and assessed for osteoclast
formation ability. In cultures containing sera from untreated celiac patients, an early
increase in the numbers of TRAP-positive multinucleated cells was observed, relative to
treatment with sera from healthy donors, which persists throughout three weeks of culture.
A lesser increase was noticed with sera from celiac patients on diet. We therefore
investigated whether different sera contained abnormal levels of specific molecules,
well-known to affect osteoclastogenesis, or, alternatively, whether they caused altered
secretion of these factors by osteoblasts. Unchanged PTH, but increased IL-6 and decreased
IL-12, were evidenced in sera from celiac patients. Nevertheless, no significant
modulations of IL-6 and IL-12 could be evidenced, by means of RT-PCR analysis, in
osteoblasts. Decreased IL-18 and osteoprotegerin (OPG) were observed, in these cells, upon
treatment with sera from untreated celiac patient, as well as increased alkaline
phosphatase activity, proliferation, and matrix mineralization. Increased proliferation
was also obtained with sera from patients on diet. This set of data demonstrates that the
osteopenia often found in celiac patients may likely be due to additional factors, beside
intestinal malabsorption, causing serum imbalance of molecules affecting bone-turnover.
[Programme]
P-118
COMPARISON OF OSTEOPROTEGERIN (OPG) AND RECEPTOR ACTIVATOR OF NF-KB
LIGAND (RANKL) BIOLOGICAL ACTIVITIES IN OSTEOCLAST CULTURES
Y. Wittrant1, S. Theoleyre1, S. Couillaud1,
C. Dunstan2, D. Heymann1, F. Rédini1*
1Laboratoire de Physiopathologie de la résorption osseuse et
thérapie des tumeurs osseuses primitives, Faculté de Médecine, Nantes, France
2Amgen Inc, Thousand Oaks, USA
Introduction : OPG and RANKL are newly discovered
molecules that are greatly involved in bone remodelling mechanisms. RANKL, expressed as a
membrane- associated protein by osteoblast/stromal cells is essential for osteoclast (OC)
differentiation/activation, and functions through its receptor RANK at the surface of
osteoclasts. OPG is an osteoblast-secreted decoy receptor that inhibits osteoclast
differentiation and activation.
Objectives : This study investigated the direct effects
of human OPG and RANKL in cultures of purified rabbit osteoclasts. Several parameters were
analysed : OC markers expression (TRAP, Cathepsin K), the activities and expression of
proteases involved in matrix bone resorption [cathepsin K, MatrixMetalloProteinase
(MMP)-9, - 2] and their Tissue Inhibitors of MMP (TIMP)-1 and -2 expressions.
Methods : Purified OC were obtained after treatment of
total rabbit bone cells with pronase/EDTA. OC were then incubated 24 hours with/without
10, 50 and 100 nanog/ml of OPG or RANKL. The expression of OC parameters was analysed by
semi-quantitative RT-PCR. The activities of MMP were analyzed by gelatin zymography. The
cathepsin K assay was performed in the presence of the synthetic fluorogenic dipeptide
Z-Leu-Arg-AMC as substrate.
Results : The expression of two OC markers (TRAP and
cathepsin K) were inhibited by 100 nanog/ml OPG, and stimulated by RANKL. Surprisingly,
the cathepsin K activity measured in the supernatants of the same cultures was inhibited
by both OPG and RANKL. MMP-2 expression and activity could not be quantified in the
majority of the assays. MMP-9 expression and activity were both enhanced in the presence
of OPG and RANKL. However, the expression of TIMP-1, the natural inhibitor of MMP-9, was
enhanced by OPG and decreased by RANKL.
Conclusion : Overall results show that RANKL exerts
pro-resorptive effects on purified OC cultures with an increase of TRAP, cathepsin K, MMP
expressions and a decrease of TIMP-1,2 expressions. In parallel, we also demonstrated that
OPG differentially regulates protease expression and activities in the same culture
condition, producing a marked inhibitory effect on cathepsin K expression (OC marker), and
a stimulation of MMP-9 activity and expression. However, this effect could be balanced by
the stimulation of TIMP-1 expression.
[Programme]
P-119
ACIDOSIS STRONGLY UPREGULATES MRNA FOR CATHEPSIN K, TRAP AND TRAF-6 IN
BONE
A. Brandao-Burch1*, S. Meghji2, T. R. Arnett1
1Department of Anatomy and Developmental Biology, University
College London, London, UK
2Eastman Dental Institute, University College London, London,
UK
Slight extracellular acidification is the obligatory
initial activation step for resorption pit formation by cultured osteoclasts (OC).
Resorption in vivo and in bone organ cultures is also highly acidosis-dependent. The great
sensitivity of OC to extracellular H+ suggests that this effect is unlikely to
be due simply to reduction of the gradient against which OC must pump H+ to
dissolve bone mineral. Furthermore, resorption pit formation involves removal of the
organic matrix of bone. To determine whether acidosis 'switches on' genes linked to
resorption, we compared mRNA expression in mouse calvarial bones maintained for 3d in
normal (pH 7.20±0.02) or slightly acidified (pH 7.05±0.02) media. Positive and negative
controls were PGE2 and OPG, respectively. Resorption was assessed as Ca 2+release.
RT-PCR was performed on DNase-treated total RNA from bones in separate reactions, using
GAPDH as control. PCR products separated by gel electrophoresis were stained with ethidium
bromide and quantified by densitometric image analysis. Values in table represent means
±SEM; n=3-5.
Thus, mRNAs for cathepsin K (a key enzyme for organic
matrix degradation by OC and TRAP5 (required for optimal resorption) were elevated
strikingly in mild acidosis. Cathepsin K mRNA was undetectable in non-resorbing bones.
Surprisingly, mRNA for c-src, thought to be required for OC ruffled border formation was
inhibited by acidosis. In parallel experiments, mRNA for TRAF-6, an intracellular factor
associated with OC activation, was elevated ~5.8-fold by similar acidosis. Cell function,
including that of osteoblasts, is normally impaired by low ambient pH. The unusual
stimulatory effect of H+ on OC may represent a primitive 'failsafe' mechanism
that evolved to correct systemic acidosis by ensuring release of alkaline bone mineral
when the kidneys and lungs are unable to remove sufficient H+ equivalent.
|
OD units |
Medium Ca2+(mmol/l) |
Treatment |
TRAP5 |
cathepsin K |
c-src |
|
pH 7.20±0.02 |
3.1±0.3 |
0 |
68.2±12.1 |
0.17±0.11 |
1microM PGE2 pH 7.26±0.01 |
16.7±7.3 |
81.7±8.5 |
151.2±7.4 |
2.60±0.21 |
pH 7.05±0.02 |
19.1±7.8 |
104.5±8.1 |
23.0±3.3 |
2.54±0.17 |
2microg/ml OPG pH 7.05±0.03 |
3.2±0.8 |
0 |
66.8±9.0 |
-0.10±0.03 |
[Programme]
P-120
OSTEOPROTEGERIN BIOLOGICAL ACTIVITY AND SIGNALLING IN OSTEOCLAST-LIKE
CELLS IS DEPENDENT ON THE PRESENCE OF RECEPTOR ACTIVATOR OF NF-KB LIGAND
S. Theoleyre1, Y. Wittrant1, S. Couillaud1,
M. Berreur1, P. Vusio2, C. Dunstan3, F. Blanchard1,
F. Rédini1, D. Heymann1*
1EE 99-01, Faculté de Médecine, Nantes, France
2INSERM U463, Nantes, France
3Amgen Inc, Thousand Oaks, USA
Osteoprotegerin (OPG)/Receptor Activator of Nuclear
Factor kB Ligand (RANKL)/RANK, three molecules recently discovered, appear to be the key
molecular triad of bone remodelling. Indeed, RANKL, expressed as a membrane- associated
protein by osteoblast/stromal cells is essential for osteoclast (OC) differentiation and
acts via its receptor RANK located at the surface of osteoclasts. In this system, OPG is
an osteoblast-secreted decoy receptor that prevents the RANKL/RANK interactions, thus
inhibiting osteoclast differentiation/activation.
More recently, we demonstrated that OPG stimulates MMP9
activity of rabbit OC by the ras/MAPK kinase pathway. The purpose of the present study was
(i) to better characterize the molecular interactions between OPG/RANKL/RANK, (ii) to
investigate the potential direct biological effect of OPG on osteoclast-like and the cell
signalling pathways associated.
OPG/RANKL/RANK interactions were studied using a BIAcore
2000 system. To examine the signal transduction pathways induced by OPG in osteoclast-like
cells, we used RAW264.7 mouse monocytes differentiated in the presence of RANKL (50
nanog/ml). Following the differentiation step, cells were cultured in serum-free medium
for 24h and were incubated with RANKL (50 nanog/ml) for 2h in the same culture conditions.
The cells were washed and incubated in the presence or absence of OPG (50 nanog/ml),
RANKL (50 nanog/ml) and anti-RANKL antibody (4 microg/ml). Cell signalling was then
analysed by western blots.
BIAcore analysis revealed that RANK and OPG bound to
RANKL charged chips with respectively Kd = 6 nanoM and 0.12 nanoM. When OPG was passed
over the RANKL-RANK association (steady-state), a response of approximately 1000 RU was
observed, demonstrating the formation of an OPG/RANKL/RANK complex. The pre- incubation of
RANKL with OPG prevented the subsequent interaction between RANKL and RANK. Concerning
cell signalling studies, RANKL induced the phosphorylation of p38 and ERK1, ERK2, in
differentiated RAW264.7 cells. Interestingly, OPG activated p38, ERK1, ERK2 pathways
whereas the co-incubation of OPG and anti-RANKL antibody abolished the p38 signal
transduction.
These original data evidence the capability of OPG,
RANKL, RANK, to form a molecular complex. Furthermore, this work describes for the first
time an OPG- induced signal-transduction pathway in osteoclast-like cells which is
dependent on the presence of RANKL via a complex OPG/RANKL/RANK.
[Programme]
P-121
CAPACITIVE CALCIUM ENTRY AND CALCIUM STORES REFILLING REGULATE
OSTEOCLASTIC SURVIVAL AND BONE RESORPTION PROCESS
R. Mentaverri*, S. Kamel, M. Brazier
UMRO, Faculty of Pharmacy, University of Picardie Jules Verne, Amiens,
France
Bone resorption is closely dependent of osteoclastic
survival and osteoclast apoptotic cell death could represent a key step at the end of this
process. The present study was undertaken to determine whether intracellular calcium store
replenishment and capacitive calcium entry (CCE) are involved in osteoclastic survival and
consequently in bone resorption. We demonstrate that i) thapsigargin (1µM), a Sarco-
Endoplasmic Reticulum Calcium ATPase pump (SERCA) blocker, decreases osteoclastic survival
from 90±1.5% to 50±1.8% (p<0.001) and bone resorption process from 100±10% to 62±8%
(p<0.001). ii) 2APB (100µM) and SKF-96365 (30µM), two store-operated channels (SOCs)
blockers, dramatically decrease osteoclastic survival and bone resorption. After 48 h of
culture with 2APB or SKF- 96365, a 75% decrease of osteoclastic survival is observed with
both compounds (p<0.001). iii) when cells are cultured in calcium-free EGTA containing
medium, rendering CCE impossible, a 50% decrease of osteoclastic survival is observed.
iiii) thapsigargin and calcium free medium inhibit synergically osteoclastic survival
which falls dramatically to a value close of 0% (p<0.001). While, osteoclastic survival
increases significantly from 50 ± 1.8% to 66.2 ± 3.7% when thapsigargin-treated cells
are cultured in the presence of 20mM of calcium, suggesting that increasing extracellular
calcium concentration stimulates osteoclastic survival when the filling of intracellular
stores is prevented by a treatment with 1 µM of thapsigargin. Taken together, our data
strongly suggest that blockade of calcium stores refilling with SERCA and CCE blockers
inhibits osteoclastic survival and consequently bone resorbing process. We suggest that in
osteoclasts, calcium movements between different cellular compartments involved in the
regulation of calcium signalling such as calcium stores refilling and CCE are closely
associated to the regulation of osteoclastic survival and that CCE may at least in part
oppose to the thapsigargin- induced osteoclastic apoptosis by a mechanism, which remains
to clarify.
[Programme]
P-122
THE ROLE OF TARTRATE-RESISTANT ACID PHOSPHATASE IN DENDRITIC CELLS
E. Esfandiari1*, C. R. Stokes1, T. M. Cox2,
M. J. Evans3, A. R. Hayman1
1School of Clinical Veterinary Science, University of Bristol,
Bristol, UK
2Department of Medicine, University of Cambridge, Cambridge,
UK
3School of Biosciences, University of Cardiff, Cardiff, UK
Tartrate-resistant acid phosphatase (TRAP) is a
histochemical marker of the osteoclast. It is also characteristic of alveolar macrophages
and dendritic cells (DCs). TRAP activity is pathologically increased in Gaucher's disease
and hairy cell leukemia, and in conditions where bone resorption is enhanced, including
osteoporosis, hyperparathyroidism and Paget's disease. DCs are the most potent
antigen-presenting cell of the immune system. They are critically involved in the
initiation of primary immune responses, graft rejection, autoimmune diseases and T cell
dependent humoral immune responses. Blood-derived human DCs matured by induction with LPS
displayed a five-fold increase in TRAP activity compared with immature DCs suggesting a
role for TRAP in the immune response.
Studies conducted with mice lacking TRAP as a result of
targeted gene disruption have an intrinsic defect of bone resorption and display an
abnormal immunomodulatory response and cytokine secretion profile in cultured bone marrow
and peritoneal macrophages. Our aim in this study was to investigate the significance of
TRAP in the dendritic cell using the TRAP knockout mice.
DCs were prepared from the bone marrow of TRAP deficient
and wild-type mice. Maturation of DCs from wild-type with LPS resulted in a significant
increase in the surface expression of MHC class II and the co-stimulatory molecule CD80.
In contrast the ability of LPS to upregulate expression of these molecules in TRAP
deficient DCs was greatly reduced. Mature DCs from TRAP deficient mice were found to
produce significantly more IL-10 and IL-12 (p40) in culture than those from wild-type
mice. The addition of anti IL-10 to cultures of LPS stimulated TRAP deficient DCs resulted
in an increased expression of MHC-II and CD80 molecules.
The role of TRAP was further investigated in vivo by
comparing the induction and expression of delayed hypersensitivity responses. TRAP
deficient and wild-type mice were sensitised and challenged with the contact sensitising
agent picryl chloride. The 24h foot pad response was significantly reduced in the TRAP
deficient mice. Together these in vitro and in vivo data would suggest that TRAP may play
a role in the maturation of dendritic cells.
Acknowledgements: Arthritis Research Campaign
[Programme]
P-123
DETECTION AND QUANTIFICATION OF RANKL AND OSTEOPROTEGERIN GENE EXPRESSION
BY REAL TIME PCR IN ILIAC BONE FROM ELDERLY PATIENTS WITH OSTEOPOROSIS AND OSTEOARTHROSIS
B. M. Abdallah1*, L. S. Stilgren3, N. Nissen2,3,
M. Kassem1,3, H. R. I. Joergensen2, B. Abrahamsen3
1Clinic for Molecular Endocrine Treatment KMEB, Odense
University Hospital, DK- 5000
2Dept of Orthopaedic Surgery O, Middelfart, Odense University
Hospital, DK-5500
3Dept of Endocrinology M, Odense University Hospital, DK-5000.
Denmark
Background: RANK-L is a potent inducer of
osteoclastogenesis and this action is blocked by the decoy receptor osteoprotegerin (OPG).
Treatment with OPG potently blocks bone resorption in postmenopausal women, but the
relationship between serum OPG levels and bone mineral density is unclear, as both
positive and negative associations have been reported in the literature. Moreover, serum
levels may not directly parallel the local production of these factors within the bone
microenvironment. Therefore, the purpose of the present study was to compare the
expression of OPG and RANK-L directly in iliac bone from patients with hip fracture
('osteoporosis') and hip replacement ('osteoarthrosis'), to test the hypothesis that the
RANK-L/OPG mRNA ratio is raised in osteoporotic bone.
Study population: 24 patients with osteoarthrosis
of the hip and six patients with hip fracture. Mean age 75.2 ±7.2 vs 77.7 ±9.1 (n.s.).
Fem. neck BMD 0.72 g/cm2 ±0.13 vs 0.57 g/cm2 ±0.09 (p<0.02). Biopsies were obtained
at the time of surgery after informed consent.
Methods: tRNA was extracted from 8 mm iliac bone
biopsies, reverse transcribed and real time quantification of RANKL and OPG to beta-actin
mRNA was performed with a SYBR Green I real time PCR assay using the comparative CT
method for calculating relative gene expression.
Results: Beta-actin normalized mRNA amounts were
as follows (median and IQ- ranges, Mann-Whitney test)
Conclusions: The median RANK-L/OPG ratio was
higher in iliac bone biopsis from patients with osteoporosis compared with patients with
osteoarthrosis of the hip, though this difference was just short of statistical
significance (p=0.09). The present study gives additional support to the involvement of
the RANKL/OPG family of cytokines in the pathogenesis of senile osteoporosis.
|
OPG mRNA |
RANK-L mRNA |
RANKL/OPG ratio |
Arthrosis |
0.005 |
0.001 |
0.175 |
| |
(0.002-0.017) |
(0.001-0.003) |
(0.120-0.421) |
Osteoporosis |
0.004 |
0.002 |
0.373 |
| |
(0.003-0.011) |
(0.001-0.005) |
(0.320-0.537) |
p |
0.56 |
0.43 |
0.09 |
Median and inter-quartile ranges |
[Programme]
P-124
DISORGANIZATION OF ACTIN-RING IN HUMAN OSTEOCLASTS WITH IMPAIRED
CATHEPSIN K EXPRESSION
S. Avnet*, A. Lamolinara, D. Granchi, A. Giunti, N. Baldini
Laboratory for Pathophysiology, Istituti Ortopedici Rizzoli, and
Department of Orthopaedic Surgery, University of Bologna, Bologna, Italy
Human cathepsin K is a cysteine protease, that highly and
selectively expressed in osteoclasts. Knockout of this enzyme in mice, as well as lack of
functional enzyme in human pycnodysostosis, result in osteopetrosis. Cathepsin K targeting
may, therefore offer as a promising tool for protection from bone loss whenever elevated
resorption
activity occurs, such as in osteolytic bone metastasis.
Despite the well-proven involvement of cathepsin K in bone resorption, different sites of
activity may be suggested for this enzyme in addition to collagen proteolysis. To
investigate the contribution of cathepsin K to cytoskeletal organization, osteoclast
polarization, and, in turn, osteoclastic bone resorption through continuous cavitation, we
studied the effects of cathepsin K completely phosphorothioate antisense
oligodeoxynucleotides (AS-ODNs) on human osteoclasts obtained from peripheral blood
monocytes. An effective uptake of AS-ODNs by osteoclasts was demonstrated by using
fluorescein- labeled AS-ODNs at different times of exposure and dosage. Treatment with
antisense significantly decreased the amount of cathepsin K in osteoclasts, as verified by
immunofluorescence and Western-blot. Under the same conditions, osteoclast viability was
unaffected, as shown by the number of TRAP-positive cells. At a10 mM dose, AS-ODN
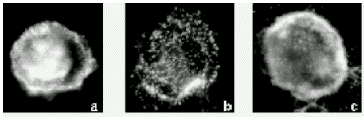
treatment inihibited pit formation, and, interestingly, also impaired the organization of
pseudopods that failed to form regular or complete actin-rings (Figure: a, negative
control, b, AS-ODN, c, S-ODN). Sense ODNs that were used as negative controls had no
effect on cathepsin K protein level, on actin-ring formation, or on resorption activity of
osteoclasts. These data show that inhibition of cathepsin K in human osteoclasts results
in a reduction of the resorption activity, associated with a derangement in the
cytoskeletal organization of the actin-ring.
[Programme]
P-125
ASSOCIATION MEMBRANE RAFTS WITH OSTEOCLAST FUNCTION
Z. H. Lee*, H. B. Kwak, H. H. Kim
Chosun University Dental College, Gwangju, South Korea
Membrane rafts are specialized microdomains enriched in
glycosphingolipids, cholesterol, and glycosylphosphatidylinositol (GPI)-anchored proteins.
Proteins modified with saturated acyl chain groups, such as GPI-anchored proteins and
double acylated proteins, and certain transmembrane proteins have been found to be
enriched in rafts. The involvement of rafts has been implicated in many important cellular
processes, which include generation and maintenance of cellular polarity, chemotactic
migration, and cell surface receptor signaling. Src family tyrosine kinases, modified by
myrisylation and palmitation, have been shown to be preferentially associated with rafts.
Given the importance of Src in bone resorbing function of osteoclasts, we investigated the
potential involvement of membrane rafts in osteoclast activity. Membrane raft microdomains
were disrupted by cholesterol depleting or sequestering agents and their effects on mature
osteoclasts were examined. We found that disruption of rafts interfere with the actin ring
formation by osteoclasts upon stimulation by receptor activator of nuclear factor kappaB
ligand (RANKL). Furthermore, dentine resorption activity of mature osteoclasts was greatly
reduced by a short exposure to the raft-disrupting agent. In addition, the survival of
osteoclasts was hampered even in the presence of RANKL when the raft integrity was
destroyed. Our findings indicate that the integrity of membrane raft microdomains is
required for proper function of osteoclasts.
[Programme]
P-126
RANKL SIGNALING TO AKT REQUIRES MEMBRANE RAFT MICRODOMAINS
H. H. Kim*, H. Ha, Z. H. Lee
Chosun University Dental College, Gwangju, South Korea
Lipid rafts are membrane microdomains, rich in
sphigolipids and cholesterol. Raft microdomains have been shown to function as a signaling
platform for immune cell surface receptors by selectively recruiting and excluding
signaling adaptors and protein tyrosine kinases to vicinity of the receptor complexes. A
few studies demonstrating raft- association of CD40, a member of the tumor necrosis factor
receptor (TNFR) family have been reported. Accordingly, tumor necrosis factor
receptor-associated factor (TRAF) 2 and TRAF3 proteins, key signaling adaptor molecules
utilized by many TNFR family receptors, were shown to be recruited to raft microdomains
during CD40 signaling. In this study we explored the possibility that lipid rafts have a
role in the signal transduction of receptor activator of nuclear factor kappaB (RANK), a
TNFR member important for osteoclast differentiation and activation. We found that TRAF6
is recruited to rafts by RANKL stimulation in osteoclasts. Furthermore, disruption of
rafts with the cholesterol depleting agents resulted in an interference in the RANKL
activation of Akt in osteoclasts. This interference by raft disruption was specific as the
MAPK and NF-kB signaling pathways were not suppressed under the same experimental
conditions. These results suggest that the membrane raft microdomain has a significant
role in RANKL signaling during osteoclast differentiation and activation.
[Programme]
P-127
AN EX-VIVO HUMAN MODEL OF OSTEOCLASTOGENESIS TO INVESTIGATE THE ROLE OF
THE PHYTOESTROGEN GENISTEIN
S. Lorenzetti*, T. Pennimpede, M. Heim, G. Kampmann, R. Scott, P. Fuchs,
P. Weber, W. Hunziker, I. Bendik, F. Branca
1National Institute for Food and Nutrition Research (INRAN),
Rome, Italy
2Roche Vitamins Ltd., Research & Development, Human
Nutrition and Health, Basel, Switzerland Hormone replacement therapy is known to have a
beneficial effect on estrogen deficiency since it stops the high rate of bone turnover
typical during postmenopause. Soy phytoestrogens have been recommended as a dietary
alternative because the soy isoflavonoid genistein has been shown to modulate osteoclast
(OCL) differentiation and resorption. As a model of human osteoclastogenesis we have taken
advantage of a recently established ex vivo approach which is based on primary cultures of
OCL hematopoietic precursors. OCL precursors are monocytes known to express CD14, CD11b
and CD11c receptors, the same markers present in macrophage precursors. By selection of
the CD14-positive monocytes is possible to isolate such precursors and, upon RANKL
induction, these cells have been shown to leave their default pathway and to be committed
as OCLs. Our aim is to investigate the role of genistein during the RANKL-induced human
osteoclastogenesis and to compare genistein effects with those of 17beta-estradiol (E2) on
the gene expression levels. We have designed TaqMan probes and primers for detecting
twenty-five different osteoclast marker genes in order to analyze the expression profiles
by quantitative real-time RT-PCR. There was an observed difference between E2 and
genistein treatment (at various concentrations) on the transcriptional regulation of some
of the investigated genes. In particular, differential regulation was observed for the
expression levels of genes encoding the osteoclast differentiation factor receptor
(hRANK), the matrix metalloproteinase 2 (MMP-2) and its inhibitor TIMP-2, the cysteine
proteinase Cathepsin K, and subunits of the vitronectin receptor (VNR).
[Programme]
P-128
SERUM TARTRATE-RESISTANT ACID PHOSPHATASE 5B AND OSTEOCALCIN IN NATURALLY
OCCURRING OSTEOPETROTIC RATS
S. L. Alatalo1*, K. K. Ivaska1, J. M. Halleen1,
S. C. Jr Marks2, H. K. Väänänen1
1Institute of Biomedicine, Department of Anatomy, University
of Turku, Turku, Finland
2Department of Cell Biology, University of Massachusetts
Medical Center, Worcester, MA, USA
Osteopetrosis is a rare, genetic disorder due to markedly
decreased bone resorption as a result of several different osteoclastic abnormalities.
Three different naturally occurring osteopetrotic rat strains have been identified;
toothless (tl/tl) containing severely reduced number of osteoclasts with normal ruffled
borders, osteopetrotic (op/op) containing slightly reduced number of osteoclasts with
poorly developed ruffled borders and incisors absent (ia/ia) containing markedly increased
number of osteoclasts without ruffled borders. The defected gene in tl/tl mutation is
colony- stimulating factor (CSF-1 or M-CSF), but the mutations in op/op and ia/ia rats are
still unknown. We measured the bone resorption marker tartrate-resistant acid phosphatase
5b (TRACP 5b) and the bone formation marker total osteocalcin from the serum of 2-
week-old osteopetrotic rats and their normal littermate controls (nlm). The results are
shown in table 1. TRACP 5b was significantly decreased and osteocalcin significantly
increased in tl/tl rats, while both markers were unchanged in op/op rats. In ia/ia rats,
TRACP 5b was significantly increased and osteocalcin unchanged. In vitro osteoclast
cultures were made from long bones of 1- to 4-day-old ia/ia rat pups. Despite of their
inability to resorb bone, osteoclasts from ia/ia rats secreted more TRACP 5b into the
culture medium than osteoclasts from nlm controls. These results suggest that in various
osteopetrosis rat strains, secreted TRACP 5b might reflect the number of osteoclasts
rather than their activity. Osteocalcin measurements suggest that bone formation is
increased in tl/tl rats but not affected in op/op or ia/ia rats.
Table 1. Serum TRACP 5b and osteocalcin
levels in tl/tl, op/op and ia/ia rats |
Mutation |
n |
TRACP 5b (U/L) (mean ±SD) |
Osteocalcin (ng/ml) (mean ±SD) |
tl/tl |
10 |
2.7 ±0.4*** |
32.7 ±2.7** |
nlm |
10 |
118.4 ±29.5 |
26.0 ±4.7 |
op/op |
4 |
100.4 ±16.2 |
27.0 ±3.5 |
nlm |
4 |
115.3 ±6.7 |
29.0 ±6.9 |
ia/ia |
7 |
482.6 ±76.1*** |
22.8 ±3.2 |
nlm |
6 |
122.2 ±18.1 |
19.7 ±3.1 |
One-Way ANOVA **p < 0.01; ***p <
0.001 |
[Programme]
P-129
CXCL5 (ENA-78) IS DIFFERENTIALLY EXPRESSED IN OSTEOCLASTS AND MONOCYTES
L. Skelton1*, A. Platt2, M. Murphy1, M.
Horton3
1Celltech R&D Ltd., 216 Bath Road, Slough, Berkshire, SL1
4EN, UK
2Celltech R&D Ltd., Granta Park, Great Abington,
Cambridge, CB1 6GS, UK
3Royal Free and University College School of Medicine, Bone
and Mineral Centre, The Rayne Institute, 5 University Street, London, WC1E 6JJ, UK
Osteoclasts are of haemopoietic origin, belonging to the
myeloid cell lineage. The importance of chemokines and chemokine receptors in the biology
of other myeloid cell types is well documented. With this in mind, a custom made
microarray was used to examine mRNA expression of chemokines and chemokine receptors, as
well as selected GPCRs, in human monocytes and 'in vitro' monocyte derived osteoclasts.
The resulting gene expression data was analysed by hierarchical clustering performed blind
to description of cell type. Data are grouped together solely on the similarity between
gene expression profiles. The analysis resulted in the formation of three clusters, one
containing four monocyte samples, one containing four osteoclast samples and a third
containing three osteoclast and two monocyte samples. The overlap of osteoclasts and
monocytes in this third cluster is representative of the similarity of expression of the
genes studied in this microarray by these two cell types. Several genes show very distinct
expression patterns. For example, CXCL5 (ENA-78) showed increased expression in the
osteoclast samples. Time course experiments for the production of CXCL5 protein in
osteoclast cultures demonstrated a correlation between levels of CXCL5 and the formation
of tartrate resistant acid phosphatase (TRAP) positive multinucleated cells.
Immunocytochemistry attributed CXCL5 production in these cultures to multinucleated
osteoclast cells. Supernatants from osteoclast cultures induced chemotaxis of peripheral
blood granulocytes and this chemotaxis could be partially blocked by antibodies to either
CXCL5 or CXCL8 (IL- 8). These data demonstrate differential expression of CXCL5 in
monocytes and osteoclasts and suggest a role for this chemokine in osteoclast biology.
[Programme]
P-130
THE INHIBITION OF OSTEOPROTEGERIN PRODUCTION IN HUMAN OSTEOBLASTIC CELLS
BY THREE GLUCOCORTICOID ANALOGUES
E. L. Humphrey1*, H. L. Smith1, J. H. H. Williams2,
M. J. Marshall1
1Charles Salt Centre, RJAH Orthopaedic Hospital, Oswestry, UK
2Chester Centre for Stress Research, Chester College, Chester,
UK
The long-term treatment of patients with inflammatory
disease using glucocorticoid drugs often results in bone loss and fractures. The mechanism
of this effect is not understood but is thought to be partly due to inhibition of
osteogenesis and partly to increased bone resorption. Bone resorption is largely
controlled by the balance between the pro-resorptive cytokine RANKL (receptor activator of
NFź-B ligand) and the decoy receptor for RANKL, osteoprotegerin (OPG).
OPG production by osteoblastic cells has been shown to be
inhibited by glucocorticoids and therefore this may lead to an increase in bone
resorption. The glucocorticoid, deflazacort (DEF), is reported to be relatively bone
sparing compared to dexamethasone (DEX) and prednisolone (PRED), that is its use results
in less loss of bone mineral density. Our aim was to see if DEF had less inhibitory effect
on OPG production in osteoblastic cells than DEX or PRED.
An enzyme-linked immuno-assay was developed to assay
human OPG produced by the osteosarcoma cell line MG63 and by primary human osteoblasts in
culture. The production of OPG by MG63 cells over 48 hours was inhibited by the three
glucocorticoids compared with vehicle control cultures. DEF showed 52 percent inhibition,
DEX showed 67 percent inhibition and PRED showed 69 percent inhibition at 10 nanomolar
concentration. When these analogues were used at dose rates that reflected the potency of
inhibition of inflammation there were no significant differences in the inhibition of OPG
production in these cells. The inhibition of OPG protein production reflected a reduction
in messenger RNA for OPG.
Thus, any bone sparing effect that DEF may have is
unlikely to be mediated through an effect on OPG production. Preliminary results with
primary human osteoblastic cells showed a similar relationship.
[Programme]
P-131
FREQUENCY OF THE P392L MUTATION IN THE SEQUESTOSOME 1 GENE IN BELGIAN
PATIENTS WITH PAGET'S DISEASE
E. Cleiren1*, G. Beyens1, J. P. Devogelaer2,
F. Vanhoenacker3, L. Verbruggen4, J. Van Offel3, W. Van
Hul1
1Dept. Medical Genetics, University of Antwerp, Belgium
2Dept. of Rheumatology, UCL, Brussels, Belgium
3Dept. of Radiology, University Hospital of Antwerp, Antwerp,
Belgium
4Dept. of Rheumatology, University Hospital of Brussels (VUB),
Brussels, Belgium
Paget's disease of bone (PDB) is a skeletal disorder
characterized by focal areas of increased osteoclastic bone resorption and disorganized
formation of new bone. It is the second most common metabolic bone disorder only preceeded
by osteoporosis. PDB is genetically heterogeneous with currently already 7 susceptibility
loci being reported.
Recently, a missense mutation (P392L) was found in PDB
cases in the gene encoding sequestosome 1 (SQSTM1) located at the PDB3 locus on chromosome
5q35. The SQSTM1 protein is a component of the NFkappaB signaling pathway, targeting
cellular proteins for degradation through the proteasome pathway. Since the mutation
affects the ubiquitin-binding domain of the protein, the hypothesis has been formulated
that decreased degradation of the SQSTM1 protein leads to increased activation of the
NFkappaB signaling pathway. The P392L mutation turned out to be recurrent as being present
in 46% of the familial cases from an extended French Canadian population but also in 16%
of the sporadic cases from the same population. Analysis of the P392L mutation in an
extended set of PDB patients, mainly from British descent, indicated that this mutation
accounts for 20% of familial cases and 8.9% of sporadic cases within the British
population.
We now evaluated the frequency of the P392L mutation in
the SQSTM1 gene in a cohorte of PDB patients of Belgian origin. By snapshot analysis
(Applied Biosystems), 92 PDB patients without any familial history related to Paget's
disease, were screened for the mutation. In only 3 patients (3.3%) the mutation was found.
This indicates that the impact of mutations in the SQSTM1 gene as the primary disease
cause is highly variable between different population and seems to be correlated with the
frequency of familial cases within the population.
[Programme]
P-132
DIVERGENT EFFECTS OF PASTEURELLA MULTOCIDA TOXIN ON THE DIFFERENTIATION
AND ACTIVITY OF MOUSE, RABBIT AND HUMAN OSTEOCLASTS
N. W. A. McGowan1*, D. Harmey1, G. Stenbeck2,
A. E. Grigoriadis1
1Craniofacial Development, King's College London, London, UK
2Bone and Mineral Centre, University College London, London,
UK
Pasteurella multocida toxin (PMT) is a
bacterially-produced toxin that causes the porcine bone resorbing disease, atrophic
rhinitis. PMT is a potent mitogen and stimulates phospholipase C, leading to activation of
protein kinase C, and increases in inositol phosphates and intracellular calcium. PMT also
signals indirectly through the Ras/MAP kinase pathway in addition to inducing actin
rearrangements through the small GTPase Rho. Previous work by our laboratory has
established that PMT inhibits osteoblast differentiation, in part via activation of Rho
and Rho kinase. Furthermore, PMT inhibits osteoblast-mediated osteoclastogenesis, partly
through a reduction in the ratio of RANKL to OPG. Despite the marked effects on
osteoblasts, the potential direct effects of PMT on osteoclast precursor differentiation
and bone resorption are less clear.
In this study, the effects of PMT on osteoclasts were
assessed in three well- established systems, sRANKL and MCSF-based cultures of murine bone
marrow cells, human peripheral blood mononuclear cells (PBMCs), and primary osteoclasts
isolated from neonatal rabbits. In murine cultures, continuous exposure to PMT dose-
dependently inhibited the formation of multinucleated TRAP positive cells and resorption
pits. Pulse experiments revealed that addition of PMT between days 1-3, 3- 6 or 6-9,
inhibited osteoclast formation and resorption. Exposure of primary mature rabbit
osteoclasts to PMT for 24h appeared to interfere with F-actin ring formation and induce
cellular contraction in some osteoclasts, although surprisingly, with no apparent effect
on resorption. In cultures of human PBMCs, PMT inhibited osteoclast differentiation and
resorption when present for the duration (0-14 days), or initial stages of culture (0-7
days). Interestingly, however, when PMT was present for the latter stages of culture
(10-14 days), there was a ~1.5-2-fold increase in the proportion of actively resorbing
osteoclasts compared to control cultures (>40% vs >70%) as assessed by expression of
vitronectin receptor and F-actin rings. This correlated with a 3-fold increase in the area
resorbed in the presence of PMT. Taken together, these findings indicate that PMT has
possible divergent effects on osteoclast differentiation and activity and we are currently
investigating the signalling pathways induced by PMT to establish their roles in
osteoclast differentiation and activity.
[Programme]
P-133
INHIBITION OF PROTEIN PRENYLATION BY RISEDRONATE CAUSES ACTIVATION OF
RAC, PAK AND P38
J. E. Dunford*, F. P. Coxon, M. J. Rogers
Dept. Medicine and Therapeutics, University of Aberdeen, Aberdeen, UK
Nitrogen-containing bisphosphonates (N-BPs), e.g.
risedronate (RIS), inhibit bone resorption by preventing the synthesis of isoprenoid
lipids required for prenylation of small GTPases such as Rho, Rac and Cdc42. Prenylation
is essential for the correct function of small GTPases since it enables membrane
localisation and interaction with regulatory factors. We sought to confirm that N-BPs
affect the activity of small GTPases in J774 macrophages, which undergo apoptosis
following treatment with N- BPs.
The active, GTP-bound forms of Rac, Cdc42 and Rho were
measured using a pull- down assay utilising agarose beads conjugated to the GTPase-binding
domain of PAK or Rhotekin. Precipitated Rac, Cdc42 or Rho was then quantitated by western
blotting. Treatment of J774 cells for 20 hours with 100microM RIS or other N-BPs caused an
increase in the level of GTP-Rac but had no effect on the level of GTP-Cdc42 or GTP- Rho.
Inhibition of Rac prenylation by 10microM GGTI-298 also caused an increase in GTP-Rac, but
clodronate (a bisphosphonate that does not inhibit protein prenylation) had no effect.
Similar results were obtained with PC3 and T47D cells.
To confirm that GTP-bound Rac in RIS-treated cells was
unprenylated, J774 cells were metabolically labelled with [14C]mevalonate in the absence
or presence of RIS. Unlike with control cells, GTP-Rac precipitated from RIS-treated cells
was not radiolabelled and hence was in the unprenylated form.
We determined next whether active, unprenylated Rac was
capable of activating downstream signalling pathways in J774 cells. Treatment for 20 hours
with 100microM RIS or 10microM GGTI-298 caused an increase in the kinase activity of PAK
immunoprecipitated from cell lysates and a sustained increase in the level of
phosphorylated p38 MAP kinase. The timecourse of p38 activation closely resembled the
activation of Rac by RIS. Inhibition of p38 with SB203580 caused an increase in apoptosis
of RIS-treated J774 cells compared to treatment with RIS alone.
These studies demonstrate that inhibition of protein
prenylation by RIS causes activation, rather than inhibition, of Rac. In J774 macrophages,
the unprenylated, activated form of Rac can activate the downstream effectors PAK and p38.
Activation of p38 may confer decreased sensitivity to N-BP-induced apoptosis.
[Programme]
P-134
NITROGEN-CONTAINING BISPHOSPHONATES INDUCE INTRACELLULAR ATP-ANALOGUE
(APPPI) PRODUCTION
H. Mönkkönen1*, S. Auriola2, J. Vepsäläinen3,
J. Mönkkönen1
1Department of Pharmaceutics, University of Kuopio, Kuopio,
Finland
2Department of Pharmaceutical Chemistry, University of Kuopio,
Kuopio, Finland
3Department of Chemistry, University of Kuopio, Kuopio,
Finland
Bisphosphonates (BPs) are a class of drugs developed for
the treatment of metabolic bone diseases with high bone turnover, such as Paget's disease,
tumor associated osteolysis and osteoporosis. Despite their widespread clinical use, the
exact biochemical mechanism of action of bisphosphonates was largely unknown, although
very recent research had shed new light on this issue.
Bisphosphonates can be divided into two classes with
distinct molecular mechanisms of action. Nitrogen-containing BPs (N-BPs), such as
alendronate and risedronate, appear to inhibit one or more enzymes of the intracellular
mevalonate pathway, thereby preventing the modification of important signalling proteins
with isoprenoid lipids. Loss of prenylated proteins causes a loss of osteoclast function
and apoptotic cell death. The less potent non-nitrogen-containing BPs (non-N-BPs), such as
clodronate and etidronate, do not inhibit isoprenylation but are metabolised
intracellularly by osteoclasts to cytotoxic analogues of adenosine triphosphate (ATP),
which accumulate in the cell cytoplasm and cause apoptosis at high doses, but has specific
anti-inflammatory action at lower doses.
It has been previously shown that N-BP:s are not
metabolised to ATP-analogue. We found, however, that N-BPs induce intracellular
ATP-analogue (Apppi) production via inhibition of mevalonate pathway in vitro by intact
mammalian cells, such as J774 macrophages and C6 gliomas. Non-N-BPs do not induce Apppi
production. The structure of Apppi (triphosphoric acid 1-adenosin-5'-yl ester 3-(3-
methylbut-3-enyl) ester) was determined by using mass spectrometry (MS) and nuclear
magnetic resonance (NMR). In contrast to the AppCp-type metabolites of non-N-BPs, Apppi
does not contain BP in the molecular structure. It seems that Apppi production correlate
the capacity of N-BPs to inhibit FPP-synthase (the enzyme of mevalonate pathway). The
potent N-BPs (such as risedronate and zoledronate) strongly inhibit FPP-synthase and also
strongly induce Apppi production. Therefore Apppi production may also correlate with
bisphosphonate activity.
[Programme]
P-135
MOLECULAR MECHANISM OF THE ACTION OF TRANSFORMING GROWTH FACTOR-BETA IN
OSTEOCLAST DIFFERENTIATION
S-J. Park*, E. J. Chang, J. S. Ko, H-M. Kim
College of Dentistry, Seoul National University, Seoul, Korea
Transforming growth factor-beta (TGF-beta) has been known
to be crucial in osteoclastogenesis that is totally inhibited when the autocrine TGF-beta
was blocked. Molecular mechanism of the TGF-beta action in osteoclastogenesis, however,
has not been studied well. In the present study, receptor activator of NF-kappaB (RANK)
signal transduction pathway was examined to understand the action of TGF- beta promoting
osteoclastogenesis. Pre-osteoclast cells attached to the surface when mouse bone marrow
cells were cultured under influence of macrophage colony stimulating factor (M-CSF) for 3
days, were further treated with M-CSF and receptor activator of NF-kappaB ligand (RANKL)
without or with TGF-beta for another 3 days to generate osteoclasts expressing RANK. Much
more multinucleated tartrate resistant alkaline phosphatase positive osteoclasts were
generated when cells were treated with M-CSF and RANKL with TGF-beta. TGF-beta treatment
kept both level of TRAF 6 and activation of NFkappa-B high upon RANKL stimulation, both of
which are known necessary conditions in osteoclastogenesis. Action of TGF- beta was
transmitted through extracellular signal-regulated kinase (ERK) 1/2 in differentiating
osteoclast cells, while smad 2 or c-Jun N-terminal kinase was not activated by TGF-beta.
These results indicate that TGF-beta may indirectly promote osteoclastogenesis by
potentiating a signal transduction pathway of RANK including TRAF6 as well as NFkappa B
which is imperative in osteoclastogenesis after activating ERK 1/2 MAPK.
[Programme]
P-136
IN VITRO VOLUMETRIC ANALYSIS OF RESORPTION PITS BY IMMUNOFLUORESCENCE
CONFOCAL MICROSCOPY
B. M. Nicholls*, G. T. Charras, M. A. Horton, S. A. Nesbitt
Bone and Mineral Centre, The Rayne Institute, University College London,
London, WC1E 6JJ, UK
In vitro bone resorption assays are essential for the
screening and analysis of bone- sparing drugs prior to their use in vivo. Herein, a novel
application of immunofluorescence confocal microscopy provides a new method that allows
individual quantification of thousands of resorption pits for number, area, depth and
volume.
Rabbit osteoclasts were seeded onto dentine slices
(pre-labelled with fluoro-X) and cultured for 3 days. A variety of resorption regulators
were used at doses to give a range of resorption while maintaining osteoclast numbers;
0.05 mM E-64 (a cathepsin K inhibitor), 0.1 mM alendronate (a bisphosphonate), 25 nM
kistrin (an integrin antagonist) and 50 pM TIMP-1 protein (a metaloproteinase inhibitor).
After fixation and permeabilisation, the cultures were analysed by immunofluorescent
staining and laser scanning confocal microscopy (LSCM) for the non-resorbed matrix with
fluoro- X and the resorption pits with Cy5-labelled antibodies to type I collagen. 16
optical xy sections were taken through the resorption sites by LSCM and the image stacks
analysed using an in-house computer programme with Pv-wave software. The fluorescence
intensity pixels for the Cy5 and fluoro-X images and their collection depths were used to
calculate pit number, area, depth and volume. Percentage changes for each of these
resorption parameters were assessed by Mann-Whitney statistics. The compounds tested gave
a range of resorption and a majority of the resorption parameters were altered
significantly (n=18, P < 0.05). Kistrin, alendronate and E-64 inhibited resorption and
reduced pit volumes by 33%, 68% and 86%, respectively, with comparable falls in pit areas.
In contrast, low concentrations of TIMP-1 proteins, increased pit volumes and areas by 88%
and 54%, respectively. Additionally, these quantification methods for bone resorption were
assessed further using the E-64 resorption inhibitor in dose response, recovery and spike
experiments. Again, significant changes in the resorption parameters were achieved.
In summary, these techniques provide an alternative,
semi-automated approach to assess in vitro bone resorption and could be further developed
to increase the throughput of screening for bone inhibitory drugs.
[Programme]
P-137
ANALYSIS OF INTRONIC POINT MUTATIONS IN TCIRG1 GENE USING MINIGENE
APPROACH
L. Susani1*, F. Pagani2, C. Stuani2, A.
Frattini1
1Istituto Tecnologie Biomediche, CNR, Milan, Italy
2Molecular Pathology Department, I.C.G.E.B., Trieste, Italy
In autosomal recessive osteopetrosis (ARO), a severe bone
disease with a fatal outcome generally within the first decade of life, a bone resorption
defect leads to increased bone density and defective remodelling. This osteoclast
malfunction causes various abnormalities including macrocephaly, progressive deafness,
blindness, hepatosplenomegaly and severe anemia.
We have analyzed more than 100 ARO patients and we found
that about 50% of them bear mutations in TCIRGB, a gene that encodes the
osteoclast-specific 116-kD subunit of the vacuolar proton pump. 37.5% of these mutations
affect the invariant GT/AG dinucleotide splicing sites. In 7 patients we identify intronic
point mutations flanking the splice sites whose effect on splicing cannot be predicted by
simple sequence inspection. Since RNAs of many of these patients may not be always
available, we evaluate the use of a specific 'minigene splicing-vector'-based approach to
study the disease-causing mechanisms.
We have analysed two mutations near the canonical GT 5'
splice site: a G to T transversion at position +4 of the intron 2 and a G to A transition
at position +5 of the intron 14. The genomic regions spanning exon 2 and exons 14, with
and without the mutations, were amplified and cloned into pTB vector (Nat Genet 2002,30
426). Wild-type or mutant minigenes were transfected in HeLa cells, the pattern of
splicing analysed by RT-PCR on extracted RNA and the resulting PCR products sequenced to
check the effect of the mutations. Both mutations caused a splicing defect. The +4 G to T
transversion caused the deletion of the last 42 bp in exon 2. Subsequent RT-PCR analysis
on patient RNA confirmed the presence of the same deletion. The +5 G to A transition,
whose patient RNA was not available, induced skipping of the entire exon 14.
Our results demonstrate that this approach is an
efficient and reliable method to test the effects of intronic point mutations when only
genomic DNA is available. We are currently evaluating the use of the minigene system to
provide mechanistic insight and possible new therapeutic strategies to correct these
splicing defects.
[Programme]
P-138
CLCN7 MUTATIONS CAUSE A BROAD SPECTRUM OF BONE DEFECTS
A. Frattini, A. Pangrazio*, L. Susani, C. Sobacchi, M. Mirolo, P.
Vezzoni, A. Villa
Istituto Tecnologie Biomediche, CNR, Milan, Italy
Infantile malignant Autosomal Recessive Osteopetrosis
(ARO, MIM 259700) is a severe bone disease with a fatal outcome, generally within the
first decade of life. Defects in bone resorption due to osteoclast malfunction is
associated with a number of severe clinical manifestations, including: macrocephaly,
progressive deafness, blindness, hepatosplenomegaly and severe anemia beginning in early
infancy or in fetal life. The only available treatment capable of providing sustained
improvement of osteoclast function is bone marrow transplantation (BMT).
In the present work, we report the clinical and molecular
analysis of a large series of 89 patients investigated for the two genes, Atp6i and ClCN7,
known to be responsible for this disease. The ATP6i (TCIRG1) codes for a subunit of the
proton pump, while the ClCN7 codes for a chloride channel. Both these molecules are
involved in the acidification of the extracellular milieu.
In our series, 48 cases showed defects in the Atp6i gene,
while 13 patients bore mutations in the chloride channel gene, while the remaining cases
did not harbour any defect in either gene.
The clinical and molecular analysis of this wide number
of patients led us to suggest that the spectrum of clinical presentation covered by these
two genes is different. Atp6i-dependent abnormalities are restricted to classical severe
ARO, and most of them are null mutations, consisting of nonsense, deletions and splicing
defects. On the contrary, the clinical spectrum of ClCN7 mutations is wider and many of
them are missense substitutions which cause severe as well as benign ADO. In addition, our
findings suggest that ClCN7 mutations are also responsible for a particular subset of
intermediate forms, showing that ClCN7 defects represent a continuum with both ARO and
ADO. Moreover, we found that the same mutation can cause a relatively broad range of
phenotypes even in the same family.
In conclusion, our findings contribute to further
highlight the heterogeneity of human osteopetroses showing that ClCN7 dependent forms
cover a wider range of clinical pictures.
[Programme]
P-139
BUTANEDIOL ESTERS AS NOVEL INHIBITORS OF BONE RESORPTION
R. J. van 't Hof*, I. R. Greig, A. I. I. Mohamed, S. H. Ralston
Dept. Medicine and Therapeutics, University of Aberdeen, Aberdeen, UK
We have recently shown that non-steroidal
anti-inflammatory drugs (NSAIDs) that contain a nitric oxide (NO)-donor group are potent
inhibitors of osteoclast activity and formation. However, the mechanism of action of these
agents is at present unknown and appears to be independent of both COX-inhibition and the
NO-donor properties. As the NO-donor group is linked to the NSAID by means of a butanediol
link, we investigated the possible anti-resorptive properties of alkane diol esters.
Co-cultures of murine osteoblasts (8x103
cells/well) and bone marrow cells (2x105 cells/well) were performed on dentine
in 96-well plates in the presence of 10 nM 1,25- (OH)2 -vitamin D3.
Drugs and IL-1beta (10U/ml) were added to the cultures on day 7 and the cultures were
terminated on day 10. Osteoclasts (OC) were identified by tartrate resistant acid
phosphatase staining, and resorption area was measured using a reflected light microscope
coupled to an image analysis system.
In the co-culture system, the butanediol ester of
biphenyl carboxylic acid (ABD56) acted as a potent inhibitor of OC formation and activity
(IC50: 23microM). When biphenyl carboxylic acid was linked to alkane diols of different
chain length (3, 5 or 6 carbons), only the hexanediol ester had some inhibitory effect on
osteoclasts, however with an IC50 >100microM. The terminal hydroxyl moiety of ABD56 is
crucial for its activity, as the biphenyl carboxylic acid ester of butanol had no effect
on OC formation or activity. To assess the importance of the biphenyl moiety for drug
potency, we tested the 1-naphthyl (4N), homoveratryl (4H), 4-biphenylacetyl (4BPA),
1-biphenylcarboxy (4BPX) and 4-phenyltoluyl (4PT) derivatives. 4BPX, 4H, 4N and 4PT were
totally inactive at concentrations up to 100 microM, and only 4BPA showed any activity
(IC50: 55microM). To assess possible inhibitory effects on bone formation, we studied the
effects of ABD56 on cultures of primary murine osteoblasts. At concentrations of up to 100
microM, ABD56 did not affect osteoblast proliferation or alkaline phosphatase expression
under both basal and PTH-stimulated conditions.
In conclusion, butanediol esters like ABD56 form a new
class of potent anti- resorptive agents with possible therapeutic use in diseases
characterized by increased bone resorption such as osteoporosis, rheumatoid arthritis and
Paget's disease of bone.
[Programme]
P-140
NO-NSAIDS INDUCE APOPTOSIS IN MACROPHAGES AND MATURE OSTEOCLASTS BUT NOT
PRIMARY OSTEOBLASTS
A. I. I. Mohamed1*, P. Del Soldato2, S. H. Ralston1,
R. J. van 't Hof1
1Dept. Medicine and Therapeutics, University of Aberdeen,
Aberdeen, UK
2Nicox SA, Sophia Antipolis, France
We have previously shown that non-steroidal
anti-inflammatory drugs (NSAIDs) that contain a nitric oxide (NO)-donor group inhibit
osteoclast (OC) activity and formation. However, the mechanism of action of these
compounds is presently unknown, and is not dependent on either COX- inhibition or NO-donor
properties. Here, we investigate whether the NO-NSAID HCT1026 and its non-nitrosylated
derivative HCT1027 induce apoptosis in rabbit OC and J774 macrophage-like cells.
Rabbit OC were isolated form the long bones of newborn
rabbits, and osteoblasts were isolated from the calvaria of newborn mice. Drugs were added
at 50 or 100 microM, and at the end of the experiments, cells were fixed and the nuclei
stained using DAPI. Apoptotic cells were identified by condensed nuclear morphology, or by
TUNEL staining. Activation of caspases was measured using the APO-ONE caspase activity kit
(Promega) in cultures of J774 cells. The profile and identity of the metabolites of
HCT1026 and HCT1027 were determined using HPLC.
Both HCT1026 and HCT1027 were potent inducers of
apoptosis in both osteoclasts and J774 cells as evidenced by numbers of apoptotic nuclei.
In J774 cultures, exposure to HCT1026 and HCT1027 resulted in DNA fragmentation detected
by the TUNEL assay and gel electrophoresis. Furthermore, HCT1026 caused a rapid (within
12h) induction of caspase-3 and -7 activity in J774 cells. Treatment of primary
osteoblasts with HCT1026 for up to 48 hours failed to induce apoptosis, suggesting that
these compounds act only on cells of the monocyte-macrophage lineage.
As cells of the monocyte-macrophage lineage express high
levels of esterases, we studied the possible metabolic breakdown/modification of HCT1026
and HCT1027. When incubated with purified esterases (0.1 microg/ml), both compounds were
rapidly hydrolysed into Flurbiprofen, the parent compound. However, in lysates from J774
cells treated with either HCT1026 or HCT1027, only the original compounds were observed,
suggesting that the activity of these drugs does not depend on the formation of
metabolites.
Our results indicate that HCT1026 and HCT1027 are
powerful inducers of apoptosis in osteoclast. However, their exact molecular mechanism of
action is currently unknown and will require further investigation.
[Programme]
P-141
PARATHYROID-HORMONE RELATED PEPTIDE EXPRESSION AT SITES OF BONE EROSION
IN RHEUMATOID ARTHRITIS
D. O'Gradaigh*, S. Bord, J. E. Compston
Bone Research Group, University of Cambridge School of Clinical Medicine,
Addenbrooke's Hospital, Cambridge, UK
Parathyroid-hormone related peptide (PTHrP), first
identified in patients with humoral hypercalcaemia of malignancy is also found in the
synovial fluid of patients with rheumatoid arthritis (RA). Its expression by
fibroblast-like synovial cells can be induced by the inflammatory cytokines TNF-alpha and
IL-1. Tumour invasion of bone is facilitated by expression of PTHrP by malignant cells
which activates local osteoclasts, the resulting bone resorption releasing TGFbeta which
then stimulates further expression of PTHrP. As rheumatoid erosion progresses relentlessly
until the joint is destroyed or surgically replaced, we hypothesised that a similar
positive- feedback loop occurred at sites of osteoclastic bone erosion in RA involving
PTHrP and TGFbeta.
We used an indirect immunoperoxidase technique to stain
serial sections of formalin-fixed paraffin-embedded RA bone and attached synovial tissue
(pannus) obtained at joint replacement surgery. Primary antibodies were a monoclonal
antibody to N-terminal human PTHrP, and rabbit polyclonal antibodies to TGFbeta isoforms
1- 3. Control sections were stained with murine IgG or pre-immune rabbit serum
respectively. Osteoclasts were identified by expression of tartrate-resistant acid
phosphatase by substrate reaction. Using image analysis software, fifteen joints were
studied, comparing the area of positive staining at the bone-pannus interface at sites of
osteoclastic erosion with non-eroded sites in the same section.
PTHrP expression was localised to the lining layer of
synovial pannus, to vascular structures in the sub-lining layer and to chondrocytes
adjacent to areas of erosion. The proportion of synovial tissue positively staining for
PTHrP was significantly higher (11.5%, sd 3.6) at sites of active erosion (as identified
by TRAP-stained osteoclasts) compared with sites where pannus was attached to bone without
erosion (6.55% sd 3.4; p=0.02). TGFbeta was not identified in the majority of sections,
with no significant differences in expression at eroded versus non-eroded sites.
In conclusion, we have found that PTHrP is specifically
associated with bone erosion in RA. As we did not identify increased expression of TGFbeta
at these sites, it is likely that PTHrP expression is instead regulated by inflammatory
cytokines in this condition.
[Programme]
P-142
HUMAN OSTEOCLAST ESTROGEN RECEPTOR EXPRESSION DIFFERS FROM THAT OF
OSTEOBLASTS
T. Pennimpede1*, S. Lorenzetti2, M. Heim1,
G. Kampmann1, R. Scott1, P. Fuchs1, F. Branca2,
W. Hunziker1, P. Weber1, I. Bendik1
1Roche Vitamins Ltd., Research & Development, Human
Nutrition and Health, Basel, Switzerland
2National Institute for Food and Nutrition Research (INRAN),
Rome, Italy
Estrogens, including the natural hormone 17beta-estradiol
(E2), exhibit effects on various tissues by binding to and transactivating the estrogen
receptor (ER). There exists extensive evidence surrounding the significance of the
preservation of healthly levels of estrogens on bone health. Investigations have shown
that estrogens are important regulators in the coupling of osteoblast and osteoclast. This
has led to speculation about the use of E2 and other estrogenic compounds to prevent and
to treat osteoporosis. Our group has previously profiled the nuclear hormone receptors in
human trabecular bone-derived osteoblast-like cells (HOBs). In this study we used
quantitative real-time RT-PCR to profile the expression levels of several isoforms of the
estrogen receptor and their splice variants, along with other members of the nuclear
hormone receptor superfamily, and various osteoclast marker genes in human osteoclast
cells (OCLs). This osteoclast cell model results from the differentiation of CD14+ cells
in the presence of receptor activator of nuclear-factor-kB ligand (RANKL) and
macrophage-colony stimulating factor. The main differences in expression levels between
hOBs and hOCLs were seen in ER-beta1, 2 and 5, peroxisome proliferator-activated receptor
gamma (PPARgamma), calcitonin receptor (CR), tartrate-resistant acid phosphatase (TRAP)
and RANKL.
[Programme]
P-143
OSTEOCLAST RUFFLED BORDER HAS DISTINCT SUBDOMAINS FOR SECRETION AND
DEGRADED MATRIX UPTAKE
M. T. K. Mulari*, H. Zhao, P. Lakkakorpi, H. K. Väänänen
Institute of Biomedicine, University of Turku, Finland
Subosteoclastic bone resorption is a result of HCl and
proteinase secretion through the late endosomal ruffled border. As bone matrix is
degraded, it enters osteoclasts' transcytotic vesicles for further processing and is then
finally exocytosed to the intercellular space. The present study clarifies the spatial
relationship between these vesicle fusion and matrix uptake processes at the ruffled
border. Our results show the presence of V-H+-ATPase, small GTPase rab7 as well as dense
aggregates of F-actin at the peripheral ruffled border, where basolaterally endocytosed
transferrin and cathepsin K are delivered. Also multivesicular body -like vesicles are
occasionally
observed to release their inner membranes to the lacuna
thus emphasizing its late endosomal nature. On the contrary, rhodamine-labelled bone
matrix enters transcytotic vesicles at the central ruffled border, where the vesicle
budding proteins such as clathrin, AP-2 and dynamin II are also localized. This mechanism
of membrane turnover appears to differ from that in neuronal synapses, where numerous
separated subdomains for membrane fusion and transmitter uptake are present. We present a
model for the mechanism of ruffled border turnover and suggest that, due to its late
endosomal characteristics, the ruffled border serves as a valuable model for studying the
dynamic organization of other endosomal compartments as well.
[Programme]
P-144
SEVERE HYPOXIA STIMULATES FORMATION BUT NOT ACTIVITY OF RODENT
OSTEOCLASTS
J. C. Utting*, I. R. Orriss, A. Brandao-Burch, T. R. Arnett
Department of Anatomy & Developmental Biology, University College
London, London, UK
Reduced O2 is well-known to act as a powerful
stimulator of the formation of marrow-derived cells. We compared the short-term effects of
hypoxia on the survival and activity of mature osteoclasts (OC) with its effects on the
formation and activity of marrow-derived OC in longer-term cultures. Mixed cell
populations containing mature OC obtained from fragmented neonatal rat bones were
sedimented onto 5mm ivory discs. The discs were cultured in sealed flasks purged with 0.2
to 20% O2 (plus 5% CO2, balance N2) in MEM / 10% FCS
acidified to pH 7.0 (1 ml / disc). After 27h, discs were fixed and TRAP-stained to enable
counting of viable, multinucleate OC; discs were restained with toluidine blue to
visualise resorption pits after OC removal. For a representative experiment, in 20, 12, 5,
2, 1 & 0.2% O2, OC numbers were 30.5±4.6, 26.2±4.0, 31.2±6.6, 16.5±2.9,
15.3±1.4 & 6.5±2.0*, respectively; numbers of pits were 87.2±13.5, 93.8±18.6,
113.3±24.4, 53.0±8.6, 68.3±8.9 & 22.3±3.4, respectively; values are means ±SEM
(n=6); * p<0.05 vs. 20% O2 value (Bonferroni). Medium pH was not altered by
O2 level. Thus, O2 levels below 5% reduce mature OC adhesion or
survival and pit formation in roughly equal measure. To study longer-term effects of very
low O2 we cultured initially non-adherent mononuclear cells from mouse marrow
on ivory discs (2x106 cells seeded / 5 mm disc) with 20 ng/ml M-CSF and 10 ng
/ml RANKL in flasks gassed as above (1 ml medium / disc). Cultures were maintained for 10d
at pH 7.3, then acidified for the final 3d, and regassed daily. For a representative
experiment, in 20, 12, 5, 2, 1 & 0.2% O2, OC numbers were 120±14, 241±48, 338±55*,
425±46**, 307±46 & 282±64, respectively; the proportion of the disc surface covered
by resorption pits was 2.8±0.3, 9.1±2.5, 18.7±3.2**, 26.6±2.1***, 21.3±4.4** &
13.1±1.4%, respectively; values are means ± SEM (n=8); *** p<0.001 ** p<0.01, *
p<0.05 vs. 20% O2 value (Bonferroni). Medium pH was not altered by O2 level.
Thus, OC formation peaks at 2% O2 but is still appreciable at 0.2% O2.
OC formed in low O2 were larger, thus hypoxia stimulated resorption more than
absolute OC numbers.
[Programme]
P-145
CALPAIN CONTRIBUTES TO THE REGULATION OF OSTEOCLAST ATTACHMENT AND
SPREADING
M. Marzia*, L. Neff, R. Chiusaroli, R. Baron, W. C. Horne
School of Medicine, Yale University, New Haven, CT, USA
The high motility of osteoclasts is thought to require
rapid transient podosome formation. Calcitonin (CT) inhibits motility, suggesting that it
might regulate at least some aspects of podosome function. CT also induces an increase in
intracellular Ca2+, which might in turn activate calpains, Ca2+-dependent
proteases that are thought to promote cell spreading and locomotion by modifying adhesion
complexes and by facilitating rear-end detachment. We therefore examined whether calpains
might be associated with adhesion structures in OCs and if CT affected calpain activity.
Immunofluorescence analysis showed high levels of mu-calpain associated with the OC actin
ring. CT treatment induced the rapid (1-3 min) dispersion of the actin ring and the
associated mu-calpain, followed soon after by cell retraction (5 min). The actin ring,
again with associated mu-calpain, reappeared 20-60 min after the addition of CT. OCs
treated with three different calpain inhibitors were less spread, and the staining of both
F-actin and mu-calpain in the actin ring was more intense than in untreated cells. In the
calpeptin-treated OCs, CT still induced the loss of the actin ring but little or no cell
retraction, and the recovery of the actin ring occurred more rapidly than in the control
OCs (3-5 min). Two known substrates of calpain, filamin A and talin were also associated
with the OC actin ring, and treatment with CT induced the dispersion of both filamin and
talin in parallel with that of the actin ring. High MW cleavage products of both filamin A
and talin and were detected by Western blotting of lysates of untreated OC, but not in
lysates of calpeptin-treated OCs, suggesting that filamin and talin may be continuously
cleaved by calpain in untreated OCs. CT treatment also transiently reduced the amounts of
filamin A and talin fragments. The demonstration that a calpain inhibitor blocks the
cleavage of the podosome-associated filamin and talin and affects the dynamic stability of
the actin ring suggest that calpain is involved in the regulation of podosome assembly
and/or disassembly. Our results also suggest that the mechanism of CT-induced retraction
of OCs may involve the transient reduction of calpain activity.
[Programme]
P-146
BIGLYCAN REDUCES LPS-STIMULATED OSTEOLYSIS BY INHIBITING OSTEOCLAST
DIFFERENTIATION
Y. Bi*, T. Kilts, X-D. Chen, T. Xu, M. F. Young
CSDB, NIDCR, NIH, Bethesda, Maryland, USA
Small leucine-rich proteoglycans (SLRPs) are
extracellular molecules that bind to collagens and growth factors to regulate cell growth
and matrix assembly. We have previously shown that biglycan (bgn), a member of class I
SLRPs, plays an important role in the differentiation of osteoblast precursors. Since
osteoblasts and their precursors regulate the differentiation of osteoclasts through
cell-cell and cell-matrix contact, we hypothesized that bgn is involved in regulating
osteoclast differentiation. To test this, we used a quantitative version of the murine
calvarial model of osteolysis to compare the effect of LPS on 7-week-old wildtype and bgn
knockout mice. Titanium particles, as a LPS-carrier, were implanted onto the parietal
bones of each mouse. After the indicated periods of time, parietal bones were harvested
and processed for x-ray using a faxitron. The extent of osteolysis in each bone was
determined from the x-ray image, in a blinded fashion, by computer-assisted
histomorphometry. Time course experiments showed that LPS induced osteolysis occurred more
rapidly and extensively in bgn knockout mice compared to wildtype mice. There was 55% less
bone resorption in wildtype mouse parietal bones than that in bgn knockout mouse parietal
bones when osteolysis reached its peak at day 7. To further understand the mechanism of
action, we determined the effects of bgn on 1,25- vitamin D3-induced osteoclast
differentiation in vitro. In those experiments, we co- cultured spleen derived osteoclast
precursors with primary calvarial cells from wild type or bgn knockout mice. Time course
and dose response experiments showed that TRAP+ multinuclear cells (MNCs) appeared earlier
and more extensively in the co- cultures containing calvarial cells from bgn knockout mice
than wildtype mice, regardless of the source of spleen cells. Thus, the inhibition of bgn
on osteoclast differentiation is mediated by calvarial cells. Further studies using PCR
analysis of mRNA from those co-cultures indicated that the increased osteoclast
differentiation might occur via a RANK/RANKL-independent mechanism. Bgn has been shown to
bind and regulate cytokines and growth factors in bone matrix, such as, TGF-beta. We
propose that bgn might inhibit osteoclast differentiation and bone resorption indirectly
by modulating production, storage and/or activity of cytokines that regulate osteoclasts.
[Hormones, Including Estrogen,
Vitamin D, PTHrP] |