| Cell Biology: Osteoblasts, Osteocytes
and Related Cytokines
P-47
RATES OF BONE FORMATION AND MINERALIZATION DURING CANCELLOUS BONE
REMODELING SIMULATED BY CHANGES IN RATES OF CELLULAR ACTIVITY AND ASSOCIATED FEEDBACK
EFFECTS
M. J. Martin*, J. C. Buckland-Wright
GKT School of Biomedical Sciences, King's College, London, UK
Predicting the effects of disruption of the bone
formation processes in the remodeling of cancellous bone could potentially accelerate the
development of pharmaceutical treatments for bone disease. We present a mathematical model
to simulate bone formation during the remodeling of healthy trabecular bone, based on the
rates of osteoblast activity and associated feedback effects within the bone
microenvironment.
Proliferation of pre-osteoblasts is described by a
relationship that characterises the growth of muscle stem cell numbers in the presence of
growth factors (Deasy et al. 2002). As proliferation is delayed until the committed
osteogenic cells are in contact with the matrix, the model incorporates the potential
effects of growth factors, from both those embedded, and those released from, the matrix.
The Michaelis-Menten kinetic equations that describe
enzyme and cellular activity, are adapted to simulate the rates of both osteoid formation
and matrix mineralization. Osteoblast activity, AObl, is simulated by the
number and activity of cells of osteoblast lineage as follows:
AObl = NObl * [(VOblmax
* [substrate]) / ([substrate] + KOblM)]
where NObl is the number of active
osteoblasts, VOblmax is the maximum rate of osteoblast activity, and KOblMis
the Michaelis-Menten constant. Two feedback effects occurring during mineralization are
included in the model: a reduction in number of active cells via osteocyte formation, and
the reduction in substrate available for mineralization as more osteoid becomes
mineralised (see Fig. 1). The model is parameterised by fitting simulations to published
data (for example Eriksen et al., 1984).
This is the first mathematical model to use the M-M
equations that describe cellular activity to simulate the rates of bone formation and
mineralization during cancellous bone remodeling.
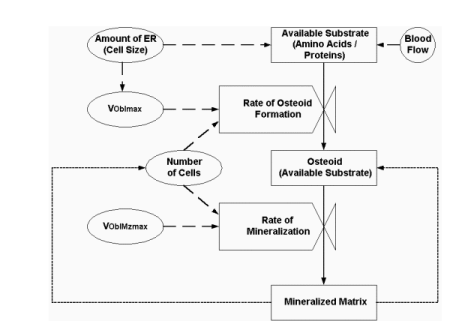
Fig. 1. Schematic diagram of the model simulating
osteoblastic activity in cancellous bone remodeling, where ER is endoplasmic reticulum, VOblmax
is the maximum rate of osteoblast osteoid formation activity and VOblMzmax is
the maximum rate of osteoblast mineralization activity. Feedback effects are represented
by dotted lines.
[Programme]
P-48
EFFECTS OF A HEPARIN-CHONDROITIN-HYALURONIC ACID LIKE POLYSACCHARIDE ON
RAT AND HUMAN OSTEOBLASTS IN CULTURE
Ph Zanchetta1*, K. Senni2, S. Igondjo-Tchen2,
G. Godeau2, N. Lagarde1, J. Guezennec3
1Laboratoire d'anaotomie pathologique, CHU morvan, 29200 brest
France
2Laboratoire de Physiopathologie des Tissus non minéralisés,
1 rue Maurice Arnoux, 92100 Montrouge, France
3DRV/VP BMH, BP 70, IFREMER, 29280, Plouzané France
The effects of a newly synthesized exopolysaccharide
(EPS) were evaluated on rat calvaria and human femoral head osteoblasts in culture. HE 800
EPS is a linear high molecular weight (800 kDa) exopolysaccharide with a repetitive
tetrasaccharidic that was used as a substitute of extracellular matrix on rat calvaria
osteoblasts and human osteoblasts based its chemical characteristics. The polymer was
added in the culture medium 10 µg/ml only one time at day 3 or each 3 days during 45
days. The effects on human femoral head osteoblasts in culture were evaluated by direct
examination, collagen type I immunodetection and Giemsa staining until days 45 in
mineralizing medium. Rat calvaria cells in culture were added with one mg of polymer in a
non mineralizating culture medium at day 1.
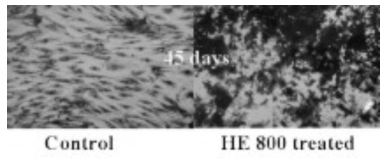
The experiments showed that HE 800 enhanced osteoblast
proliferation and phenotypic expression on both rat and human osteoblasts. Mineralization
appeared as early as day 3 or 5 depending of the culture medium. Cells were rapidly
organized in a three-dimensional network and after 45 days of culture cells were entirely
covered by mineralized deposit while control culture cells were perfectly identifiable
(Graphic). Collagen type I expression in human osteoblasts began after 15 days and was
both intra and extracellular. These experiments confirmed the results obtained in vivo
with a model of bone healing in rat calvaria created defects.
[Programme]
P-49
OSTEOCYTES STIMULATE THE PROLIFERATION AND DIFFERENTIATION OF OSTEOBLASTS
IN VITRO
T. J. Heino*, T. A. Hentunen, H. K. Väänänen
Institute of Biomedicine, Department of Anatomy, University of Turku,
Turku, Finland
Osteocytes are the most abundant cells in bone. Their
distinct morphology and networking suggest that they play a specific role in the
regulation of bone homeostasis. MLO-Y4 is a primary osteocyte-like cell line, which
possesses many of the properties of primary osteocytes. It was developed by targeting SV40
large T- antigen oncogene into osteocytic lineage using osteocalcin promoter. We have used
MLO-Y4 cells to identify potential signals between osteocytes and other bone cells. MLO-Y4
cells were cultured near to confluency and the medium was changed into serum-free
alpha-MEM. MC3T3-E1 cells were used as a control. The conditioned medium (CM) was
collected after 24 hours, centrifuged and kept at +4 deg C. Mouse osteoblasts were
isolated from the bone marrow of 10 week-old female NMRI mice and cultured in the presence
of dexamethasone, ascorbic acid and sodium beta- glycerophosphate. After 7 days of
culture, subcultures were prepared and continued in
the presence of MLO-Y4 CM. On days 16 - 18, the ALP
activity in the cultures was assayed and on day 21, the amount of deposited calcium was
measured. Osteocyte CM increased the ALP activity in osteoblast cultures (36 and 77% with
10 and 20% CM, respectively) and effect on the calcium deposition was even more pronounced
(134 and 262% with 10 and 20% CM, respectively). MC3T3-E1 CM did not have any stimulatory
effects. To study the possible pro-proliferative and anti-apoptotic effects of osteocyte
CM on osteoblasts, proliferation and viability assays were performed. The proliferation of
osteoblasts was increased 1.5-fold in the presence of osteocyte CM. On the basis of serum
starvation experiments, we concluded that MLO-Y4 CM does not protect osteoblasts from
apoptosis. This suggests, that the factor(s) in osteocyte CM are not anti-apoptotic, but
rather pro-proliferative, stimulating the differentiation and bone formation capacity of
osteoblasts. We have excluded some possible factors and are now in the process of
analyzing the factor in more detail. In conclusion, MLO-Y4 osteocytes secrete factors that
stimulate osteoblast proliferation, differentiation and function. Osteocyte CM does not
have survival effects on osteoblasts during serum starvation, but has a strong stimulatory
effect on bone formation.
[Programme]
P-50
MEPE PROTEIN IS HIGHLY EXPRESSED IN OSTEOCYTES IN HUMAN BONE
A. Nampei1*, J. Hashimoto1, K. Hayashida1,
H. Tsuboi1, K. Shi1, H. Miyashita2, T. Yamada2,
S. Morimoto3, T. Ochi1, H. Yoshikawa1
1Department of Orthopaedic Surgery, Osaka University Graduate
School of Medicine, Osaka, Japan
2Pharmaceutical Research Division, Takeda Chemical Industries,
Ltd., Osaka, Japan
3Department of Geriatric Medicine, Kanazawa Medical
University, Kanazawa, Japan
Matrix extracellular phosphoglycoprotein (MEPE) gene is
highly expressed in tumors which cause oncogenic hypophosphatemic osteomalacia (OHO). MEPE
is also known to be one of the bone-tooth matrix proteins, which might be one of the
inhibitory factors of bone mineralization. We have performed cloning of human MEPE gene
from cDNA library of human nasal tumor causing OHO. Then we have obtained recombinant
human MEPE protein (rhMEPE) and developed a rabbit polyclonal antibody against rhMEPE. We
have confirmed that anti-rhMEPE antibody, thus obtained, has been available for detection
of the rhMEPE expressed in E.coli, CHO and insect cells. Using this polyclonal antibody,
we analyzed the distribution of MEPE protein in human bone by immunohistochemistry. The
data from four normal control people revealed that MEPE protein was predominantly
expressed in osteocytes, including their dendritic processes and pericellular bone matrix
but not by osteoblasts or bone-lining cells. However the bone specimen from OHO patient
revealed that MEPE was focally expressed in deeply-located osteocytes. Then we have
compared the MEPE positivity of osteocyte between mineralized area and non- minerarized
osteoid area using serial sections from undecalcified bone, which were embedded in
methylmethacrylate, obtained from four osteomalacia and four osteoporosis patients.
Osteomalacia patients consisted of two OHO, one Fanconi's syndrome, and one vitamin
D-deficient rickets. For osteomalacia MEPE positivity of osteocyte in mineralized bone is
87.5 ±8.6 % and that in osteoid is 7.8 ±6.4 %, meanwhile for osteoporosis MEPE
positivity of osteocyte in mineralized bone is 95.3 ±0.5 % and that in osteoid is 4.9
±5.7 %. In specimens from osteomalacia patients, regardless of the cause of osteomalacia,
MEPE protein was mainly localized in osteocytes embedded in the matrix of mineralized
bone. Similar results were obtained with samples from osteoporosis patients. Our data
provide the first evidence that MEPE protein is expressed by osteocytes in human bone
tissue and MEPE positive osteocytes are predominantly localized to mineralized bone.
[Programme]
P-51
CLINICAL STUDY ON BONE RESORPTION BIOCHEMICAL MARKER OF SECOND
GENERATION-SERUM TARTRATE- RESISTANT ACID PHOSPHATASE(TRACP)5B
Q. Xiang1*, N. Su1, Z. H. Liu1, D. Q.
Yin2, H. M. Zhu3, S. Y. Chen3, Z. L. Tan4,
L. Wang4, J. H. Lu5, Y. J. Qin5
1China-Japan Friendship Hospital, 100029, Beijing, P.R.China
2Beijing JiShuiTan Hospital, P.R.China
3Shanghai North China Hospital, P.R.China
4Tianjin Hospital, P.R.China
5The Sixth Hospital of Shanghai, P.R.China
Osteoporosis is the major cause of morbidity and
mortality in the elderly population. Early diagnosis of osteoporosis are concerned by
scientists. A problem with the first generation markers of bone resorption is that their
normal levels vary a lot between individuals. Although these markers are useful in
selecting and mornitoring antiresorptive treatment and in the prediction of osteoporotic
fractures, it is difficult to be a biomarker of osteoporotic diagnosis. The novel study
showed that tartrate-resistant acid phosphatase(TRACP) 5b maybe a good second generation
biomarker of osteoporosis.
The serum of 437 person were detected with Bone TRAP kits
in several hospitals of China. Our data implied that serum TRACP5b level of osteoporosis
patients and older person (man>or=65, woman>or=50) are higher than normal. It will
be a convenient, reliable and economical new method for determination for bone resorption
rate from serum samples.
Our data showed that the serum TRACP5b level were lower
in men before 64 and in women before 49 years old respectively. But their TRACP5b level
and bone resorption, especially in osteoporotic patients, were increased climacterum
later, and there are markedly differentiation compared with adult and control groups in
statistics. These results implied that the serum TRACP5b may be a more specific marker in
mornitoring bone resorption and osteoporosis diagnosis.
|
Age |
No. |
TRACP5b value |
t-test |
| |
|
|
X ±SD |
|
|
Normal male |
20-64 |
87 |
3.76 ±1.35 |
|
|
Normal male |
> or =65 |
79 |
4.66±1.50 |
*p<0.01 |
|
osteoporotic male |
> or =60 |
58 |
5.69±1.86 |
*p<0.01 |
**p<0.01 |
Normal female |
20-49 |
73 |
2.89±1.28 |
|
|
Normal female |
> or =50 |
115 |
4.81±1.54 |
#p<0.01 |
|
osteoporotic female |
> or =45 |
61 |
5.24 ±1.65 |
#p<0.01 |
##p<0.05 |
*:compared with 20-64 group;**:compared with
>or=65group;#:compared with 20-49group;##:compared with >or=50group |
[Programme]
P-52
MEMBERS OF THE TGFBETA SUPERFAMILY ANTAGONIZE DIFFERENTIALLY THE
INHIBITORY ACTIVITY OF BONE MARROW EXTRACELLULAR FLUID ON OSTEOBLAST-LIKE CELLS
PROLIFERATION
D. Egrise1*, P. Bergmann2, A. Schoutens1
1Nuclear Medicine, Erasme Hospital, Brussels, Belgium
2Clinical Chemistry, CHU Brugmann, Brussels, Belgium
We have shown previously that rat bone marrow
extracellular fluid (BM supernatant) inhibits in vitro the proliferation of
osteoblast-like cells, suggesting a possible negative regulation of bone formation by bone
marrow microenvironment. The inhibitory power of BM supernatant increases progressively
with age and correlates with the extent of trabecular bone loss. Inhibition could not be
attributed to one of the many cytokines known to inhibit osteoblast proliferation or bone
formation like IFNgamma,IGFBP-4,IL-1,IL-4,TNFalpha,BMP-3In this work, we hypothesized that
the inhibiting factor(s) in the BM supernatant could be related to one of the recently
described proteins which inhibit BMP's activity through binding them. Thus, we examined if
the inhibiting power of BM supernatant could be modulated in vitro by members of the
TGFbeta superfamily.
Preincubation of the BM supernatant for two hours with
increasing concentrations of rhBMP-2(0.05-1.25 microg/ml) reversed dose-dependently its
inhibitory effect on the proliferation of osteoblastic cell lines (ROS 17/2.8 and Saos2)
and of primary osteoblasts derived from bone marrow or trabecular bone. Preincubation of
the BM supernatant with rhBMP-6(0.05-1.25 microg/ml) had no effect on its capacity to
inhibit proliferation in all the culture models tested. Preincubation of the BM
supernatant with rhTGFbeta(5-100 nanog/ml) improved more efficiently the proliferation of
bone marrow and trabecular bone-derived cells than those of osteosarcoma cell lines.
Preincubation of the cells for two hours with the cytokines before medium change and
incubation with the BM supernatant did not modify its capacity to inhibit cell
proliferation.
The neutralization of the inhibitory capacity of BM
supernatant by BMP-2 and TGFbeta suggests that the factor(s) responsible for the
inhibition could belong to a family of proteins known to antagonize BMPs activity by
binding them with high affinity, like noggin,chordin and the DAN/cerberus family of genes.
Some of these proteins have been shown recently to play a role in the regulation of bone
formation. The differential expression of these proteins in the bone marrow of young and
aged rats will be studied by DNA microarrays.
[Programme]
P-53
PLATELET-DERIVED GROWTH FACTORS ENHANCE PROLIFERATION OF HUMAN STROMAL
STEM CELLS
E. Lucarelli1*, A. Beccheroni1, A. Cenacchi2,
A. M. Del Vento2, L. Sangiorgi1, A. Zambon Bertoja1,
P. Picci1, M. Mercuri3, P. M. Fornasari2,4, D. Donati3,4
1Oncological Research Laboratory
2Blood Transfusion Service
3V Division of Orthopedic Surgery
4Musculoskeletal Tissue Bank
Cultured stromal stem cells (SSC) are an in-vitro bone
cell model that have great revelance to osteoporosis research and drug discovery. It is
important to develop culture conditions for ex-vivo expansion of SSC that do not
compromise their self- renewing and differentiation capability. Bone marrow SSC and
platelet gel (PG) obtained by platelet rich plasma provide an invaluable source for
autologous
progenitor cells and growth factors for bone
reconstruction. In this study the effect of platelet rich plasma (PRP) released by PG on
SSC proliferation and differentiation was investigated. MTT assay was used to investigate
the effect of PRP on proliferation: results showed that PRP induced SSC proliferation.
However, the effect was dose dependent and proliferation was induced when cells were
cultured in 10% PRP. Untreated cells served as controls. Upon treatment with 10% PRP,
cells entered logarithmic growth by day 6, and by day 9 there were 7 times more cells
compared to controls. Removal of PRP restored the characteristic proliferation rate.
Because SSC can gradually lose their capacity to differentiate along the osteogenic
lineage during subculture in-vitro, we tested whether 10% PRP treatment affected SSC
osteogenic potential. SSC were first treated with 10% PRP, after 6 days PRP was removed
and cells were treated with osteogenic supplements (DEX). DEX induced a 3-fold increase in
the number of alkaline phosphatase positive cells and induced mineralization that is
consistent with the differentiation of osteoprogenitor cells. In conclusion, 10% PRP
promotes SSC proliferation. Cells expanded with 10% PRP can differentiate along the
osteogenic lineages once PRP are withdrawn, producing mineralised extracellular matrix.
[Programme]
P-54
WITHDRAWN
[Programme]
P-55
ULTRASTRUCTURAL AND MOLECULAR STUDY OF OSTEOBLAST FUNCTION IN XENOPUS
LAEVIS AND ANAS PLATYRHYNCHOS DURING THE ANNUAL CYCLE
A. Quilhac*, J. Castanet
UMR 8570 Systèmes ostéo-musculaires, Université Paris VI, MNHN,
Collège de France, Paris, France
In bony tissues, structural modifications occur during
ontogenesis and during the annual cycle. These modifications mainly result from changes in
the osteoblast function, cells which synthetize the organic matrix. Many studies deal with
osteoblast function in vitro but few show the cellular and molecular modifications
associated with osseous matrix synthesis in vivo. We have undertaken experiments to
understand how the osteoblast population participated in the elaboration of the bony
tissue and to search correlation betwen cyto-morphological features and spatio-temporal
gene expression during osteoblast activity.
As amphibians and birds present typical differences in
bone growth and structure, we have chosen the long bones of Xenopus leavis and Anas
platyrhynchos as models to add a comparative aspect to this study. This choice was
supported by previous observations suggesting that, while osteoblast undergo annual cycle
of activity and inactivity in Xenopus, these cells are active during the whole
annual cycle in Anas.
We report here a descriptive study using transmission
electron microscopy (TEM) and in situ hybridization which correlate osteoblast
activity with the expression of genes known to be involved in bone growth and
differentiation as Bmp4 and Dlx5.
[Programme]
P-56
PROTEIN KINASE D INDUCED ACTIVATION OF MAP KINASES JNK AND P38 IS A NEW
SIGNALING PATHWAY INVOLVED IN BMP-2 INDUCED OSTEOBLASTIC CELL DIFFERENTIATION
J. Lemonnier, Ch. Ghayor, J. Caverzasio*
Division of Bone Diseases, University Hospital of Geneva, Geneva,
Switzerland
BMP-2 is a critical morphogenetic protein for the
development of osteoblastic cells. The cellular mechanisms by which BMP-2 induces this
process remains, however, uncompletely understood. Recent findings suggest that, in
addition to the SMAD pathway, BMP-2 may also signal via protein kinase C (PKC) and/or the
mitogen-activated protein kinases (MAPKs) JNK and p38. In this study, we investigated the
molecular mechanism by which BMP-2 activates JNK and p38 and the role of these pathways in
the differentiation of osteoblastic cells.
In MC3T3-E1 osteoblastic cells, the activation of JNK and
p38 by BMP-2 (23-25 x at 3h) was not affected by overnight pretreatment of the cells with
PMA suggesting that conventional PKC isoforms are not involved in this signaling response.
In contrast, the PKC and protein kinase D (PKD) inhibitor Go6976 (10 µM) completely
blocked BMP-2-induced activation of JNK and p38 without influencing SMAD1,5
phosphorylation. Interestingly, Go6976 also completely blunted the stimulation of alkaline
phosphatase (ALP) activity (3-5x) and of osteocalcin (Oc) production (20- 25x) induced by
BMP-2. PKD is a newly described diacylglycerol-sensitive protein kinase with homology to
PKCs and unknown function. Among various PKCs expressed in MC3T3-E1 cells, BMP-2 only
induced phosphorylation/activation of PKD with a maximal effect (3.3x) after 1 h
incubation and this response was completely prevented by Go6976. To further determine the
role of PKD in mediating activation of JNK and p38 by BMP-2, we constructed MC3T3-E1 cell
lines stably expressing PKD antisens oligonucleotide (AS-PKD). In these cell lines, PKD
expression was lowered by 50-60% compared with vector transfected cells (V-PKD).
Interestingly, activation of JNK and p38 as well as the stimulation of ALP and Oc induced
by BMP-2 were markedly impaired in AS-PKD compared to V-PKD transfected cells suggesting a
functional role of activation of JNK and p38 by PKD in osteoblast-like cells.
In conclusion, our data describe a new signaling pathway
activated by BMP-2 in osteoblastic cells. This pathway involves PKD-dependent activation
of JNK and p38 which, in addition to SMADs, appears to be essential for the effect of
BMP-2 on osteoblastic cell differentiation.
[Programme]
P-57
GLOBAL GENE EXPRESSION STUDY OF MLO-Y4 CELL LINE USING TALEST (TANDEM
ARRAYED LIGATION OF EXPRESSED SEQUENCE TAGS)
H. J. Vuorikoski*, K. J. Büki, H. K. Väänänen
Institute of Biomedicine, Department of Anatomy, University of Turku,
Turku, Finland
The recently finished genome projects gave us the access
to the full genome, but monitoring the gene expression profiles reguires techniques that
are valid both in qualitative and quantitative level. A number of methods have been
developed to asses and quantify gene expression. Classic techniques like northern blotting
are not suited for generating global gene expression profile. At the moment the most
widely used method for parallel analysis of global gene expression for known genes is
high- density microarrays. But the method is still restricted to number of genes that can
be analysed on a single chip and it is restricted to only to genes whose sequence is
already available. These restrictions can be overcome with techniques like SAGE or TALEST.
We used TALEST (Tandem Arrayed Ligation of Expressed Sequence Tags) to generate global
gene expression profile of mouse MLO-Y4 cell line. This osteocyte-like cell line,
described 1997 by Kato et al., was established from transgenic mice and is a useful tool
to study osteocyte function. We extracted mRNA from cultured MLO-Y4 cells, and after cDNA
synthesis performed the TALEST as described by Spinella et al. Among the 542 tags
sequenced, 89 (16,4%) appeared more than once. Seventy-six of these appeared more than
three times. The most abundant mRNA was beta-actin, which consisted 4,6% of the total mRNA
population. Except for mitochondrial or ribosomal genes, the next abundant genes were
Mpv17- like protein (3,1%), calcyclin (2,9%) and dipeptidyl peptidase 8 (2,4%). Some of
the most abundant genes were also two still unknown genes, one consisting 3,1% and the
other 2,1% of total mRNA population. Even though the expression profile survey of MLO-Y4
transcripts we made is limited, it reveals genes expressed in this osteocyte- like cell
line. The expression profile gives us a baseline for further studies.
[Programme]
P-58
PLASTICITY IN ADIPOGENIC AND OSTEOGENIC DIFFERENTIATION PATHWAYS
ORIGINATING FROM HUMAN BONE MARROW DERIVED MESENCHYMAL STEM CELLS
N. Schuetze*, U. Noeth, J. Schneidereit, J. Eulert, F. Jakob
Orthopaedic University Hospital, Molecular Orthopaedics, Wuerzburg,
Germany
Expansion of adipose tissue in bone marrow at the expense
of osteogenesis is age- related and may contribute to diseases like osteoporosis and
osteonecrosis. The molecular basis for this phenomenon is largely unknown and both
differentiation pathways are incompletely understood. We established a cell culture system
which allows for reprogramming (transdifferentiation) of osteoblasts into adipocytes and
vice versa during differentiation pathways originating from human mesenchymal stem cells
(hMSCs). Cells were isolated from the femoral head of patients undergoing total hip
arthroplasty. For transdifferentiation of osteoblasts into adipocytes hMSCs were cultured
in osteogenic medium for 2 weeks. Alkaline phosphatase (AP) staining revealed a homogenous
increase in expression. Osteocalcin and AP were highly expressed by RT-PCR analysis. These
committed osteoblasts were then cultured in the adipogenic medium for 2 weeks in order to
induce the transdifferentiation (reprogramming). After 2 weeks cells displayed a
homogenous oil-red O staining. Direct adipogenic differentiation of hMSCs performed in
parallel displayed the same homogenous staining. After adipogenic transdifferentiation of
the committed osteoblasts no RNA markers for osteoblasts remained detectable whereas the
lipid markers (lipoprotein lipase and peroxisome proliferator activated receptor gamma2)
were highly expressed. For transdifferentiation of adipocytes into osteoblasts the hMSCs
were cultured in adipogenic medium for up to 2 weeks which was controlled by analysis of
appropriate marker expressions in RT PCR analyses and stainings. Thereafter cells received
the osteogenic medium for 4 weeks resulting in marked deposition of mineral (alizarin red
staining) and expression of osteoblast markers in RT-PCR analysis. Some adipocytes
however, did not respond to the osteogenic medium since residual lipid markers were still
detectable. Our results indicate that the plasticity between osteogenesis and adipogenesis
extends during the differentiation pathways up to the terminal differentiation stage of
osteoblasts and adipocytes. The transdifferentiation process of committed osteoblasts into
adipocytes was as efficient and followed the same kinetics as the direct differentiation
of adipocytes from hMSCs. Since the molecular mechanisms of this reprogramming phenomenon
are unknown cell culture systems could provide the basis for the elucidation of the
underlying molecular pathways. Thereby, novel targets could be detected for therapeutic
interventions in order to stimulate osteogenesis.
[Programme]
P-59
EXPRESSION OF PRE-OSTEOBLAST MARKERS AND CBFA-1 AND OSTERIX GENE
TRANSCRIPTS IN STROMAL TUMOR CELLS OF GIANT CELL TUMOR OF BONE
L. Huang1*, Y. Y. Cheng1, X. Y. Teng1,
K. M. Lee2, S. M. Kumta1
1Dept. of Orthopaedics & Traumatology, the Chinese
University of Hong Kong
2Leehysan Clinical Research Laboratory, the Chinese University
of Hong Kong
ABSTRACT
In giant cell tumor of bone (GCT), the mononuclear
stromal cells represent neoplastic component of this tumor and regulate the formation of
multinucleated osteoclast-like giant cells. However, the origin of stromal tumor cells has
not yet been clearly defined. In this study, we have initially evaluated several
osteoblast markers, collagen type I, BSP, osteonectin and osteocalcin in GCT stromal tumor
cells by immunohistochemistry. We have further examined the gene expression of Cbfa-1 and
Osterix, two key transcription factors required for osteoblast differentiation, and gene
expression of osteocalcin and ALP in GCT stromal tumor cells. We have also determined the
regulation of these genes expression by BMP-2 using Real-Time Quantitative RT-PCR
analysis. Among the thirteen cases of GCT specimens and seven GCT stromal cell cultures
studied, the results showed that majority of GCT stromal cells synthesize type I collagen,
BSP and osteonectin, but not produce differentiated osteoblast marker, osteocalcin. We
also described for the first time the expression of low extent Cbfa-1 and Osterix mRNA in
GCT stromal tumor cells. In cultures, the GCT stromal tumor cells were indicated response
to BMP-2 by the up-regulation of Cbfa-1 and Osterix gene expression, and the increase of
osteocalcin mRNA level. ALP activities were also significantly increased following culture
in the presence of BMP-2. In summary, our data suggest that GCT stromal tumor cells might
descent from osteoblast lineages and retain the ability for differentiation.
[Programme]
P-60
CENTRAL PERFUSION OF LEPTIN DECREASES BONE FORMATION IN THE RAT BUT DOES
NOT INCREASE THE POWER OF BONE MARROW EXTRACELLULAR FLUID TO INHIBIT OSTEOBLAST
PROLIFERATION
D. Egrise1*, A. Lubansu2, A. Schoutens1,
P. Bergmann3
1Nuclear Medicine, Erasme Hospital, Brussels, Belgium
2Neurosurgery, Erasme Hospital, Brussels, Belgium
3Clinical Chemistry, CHU Brugmann, Brussels, Belgium
Leptin perfusion in the third ventricle of mice induces
bone loss by decreasing osteoblast activity. The sequence of events leading from the
hypothalamus to the osteoblast is still largely unknown. A modification in the bone marrow
microenvironment could be one of the last events of the sequence leading to the decreased
bone formation. We have shown that rat bone marrow extracellular fluid (BM supernatant)
inhibits in vitro the proliferation of osteoblast-like cells and that this inhibitory
capacity increases with age and is correlated with trabecular bone loss. The aim of this
study was to assess the hypothesis that the decreased bone formation induced by a central
perfusion of leptin could result from modifications in bone marrow microenvironment
leading to a higher capacity of the BM supernatant to inhibit osteoblast proliferation.
Seven weeks old male Wistar rats received an
intracerebroventricular perfusion of leptin (8 nanog/hour) for 3,10 or 14 days. Rats were
injected with calcein at days 1 and 9 after the perfusion was started. The weight of the
rats receiving leptin was lower than that of control animals at days 10 (275 ± 11 vs 303
± 3 g) and 14 (306 ± 4 vs 337 ± 9). At day 10, bone formation was markedly decreased in
the rats which had received leptin, as assessed by the percent double labeled surface
measured in the secondary spongiosa of the lower femoral metaphysis (4.3 ± 1.8 % in rats
perfused with leptin vs 16.4 ± 2.6 % in controls). As observed in the mouse, the
osteoblastic index was not affected by leptin perfusion. Bone surface was not
significantly modified (9.03 ± 1.5 vs 9.84 ± 1.49 mm/mm2 analyzed surface) at day 10.
The BM supernatant of rats perfused with leptin for 3 or
10 days had a similar inhibitory capacity on osteoblast proliferation than that of control
rats.Ex vivo cultures showed that the number of osteoprogenitors was not affected after a
14 days perfusion of leptin, nor their capacity to differentiate and to mineralize in
vitro. These results confirm in the rat that leptin has a central regulating effect on
bone formation, independent of osteoblast number, as in mice. They show that this action
does not result from an inhibition of osteoblast proliferation by a change in the bone
microenvironment.
[Programme]
P-61
NERIDRONATE AND OSTEOPOROTIC AND OSTEOARTHRITIC HUMAN OSTEOBLASTS
A. Corrado*, L. Quarta, N. Melillo, F. P. Cantatore
Rheumatology Unit , University of Foggia, Italy
Bisphosphonates (BFs) are currently used for treatment of
diseases characterized by an elevated bone turnover. The main pharmacological effect of
BFs is the inhibition of osteoclasts, but the exact mechanism of action BFs is not still
understood. These compounds can inhibit osteoclast activity both directly than indirectly
trough osteoblasts. Several studies indicate that osteoblasts could be the alternative, or
even the main target cells for bisphosphonates, although their effects on osteoblastic
metabolism are different and often contradictory. The aim of this study is to evaluate the
metabolic in vitro effect of the bisphosponate Neridronate on normal and pathological
human osteoblasts.
Pathologic osteoblasts were obtained from subchondral
bone of patients undergoing to total knee joint replacement for osteoarthritis (OA) and
total hip replacement for osteoporotic (OP) fractures. Normal human osteoblasts were
isolated from cancellous bone specimens of healthy subjects undergoing surgery for
traumatic femoral fractures. We evaluated osteocalcin (GLA) production by OA, OP and
normal osteoblasts in presence of Neridronate 10-4M and 10-6M before
and after Vitamin D stimulation.
We found that, compared to normal osteoblasts, GLA
synthesis was significantly greater in OA osteoblasts whereas it was significantly lower
in OP osteoblasts, confirming the different metabolic activity of these cells; GLA
synthesis was significantly increased by Vitamin D stimulation in all cells. Both in
normal and in pathological osteoblasts, treatment with higher Neridronate concentration
(10-4M) induced a significant reduction of GLA synthesis compared to cells
without any treatment; further, an inhibition of Vitamin D induced GLA production was
observed. The stimulation with lower Neridronate concentration (10-6M)
determined a significant increase in GLA production in normal and OP osteoblasts; this
effect was strongly enhanced by Vitamin D in normal osteoblasts. Conversely, in OA
osteoblasts, Neridronate 10-6M did not induce an increase in GLA synthesis and
exerted an inhibitory effect on Vitamin D stimulation.
These data suggest that Neridronate can enhance or
decrease metabolic osteoblastic activity, both in normal and pathological conditions, with
an inhibitory or stimulatory effect on Vitamin D response, depending on compound
concentration and different cell types.
[Programme]
P-62
MODULATION OF IGF-1, OSTEOPONTIN AND BCL-2 GENE EXPRESSION IN RAT TIBIA
BY MECHANICAL LOADING
H. W. van Essen*, A. M. Tromp, P. Lips, N. Bravenboer
Research Institute for Endocrinology, Reproduction and Metabolism, VU
Medical Center, Amsterdam, The Netherlands
Introduction: One of the major factors determining
bone mass and bone structure is the daily habitual mechanical loading of the body. In this
process the regulation of osteocyte cell survival and cell death seems to play an
important role. Verborgt et al recently described a regulatory role for osteocyte
apoptosis after bone fatigue loading (JBMR 2002;17:907-914). We have investigated the
influence of physiological mechanical loading on the expression of Bcl-2, an
anti-apoptotic member of a family of regulatory proteins, Osteopontin and IGF-1, in a
pilot experiment using real-time RT-PCR.
Methods: One year old female rats were divided in
six groups of three rats. The right tibia of the rats was loaded in the 4-point bending
apparatus (300 cycles, 2 Hz, 60 N) while the left tibia was sham loaded. At the time
points 3, 6, 16, 24 and 48 hours after loading the rats from a group were sacrificed. The
tibiae were dissected and frozen in liquid nitrogen. In a separate experiment we used six
rats as controls which were not loaded at all. RNA was extracted from the tibia shaft and
gene expression was measured with real-time RT-PCR using specific primers and fluorescent
probes for Bcl-2, Osteopontin and IGF-1. Expression was normalized against the expression
of the housekeeping gene PBGD. Expression in the loaded or right tibia was calculated
relative to the sham loaded or left tibia. Because this was a pilot experiment with three
rats per group no statistical analysis was performed.
Results: There is a two-fold variation in the
basal expression of these genes in the control rats. There was a slight but reproducible
increase in IGF-1 gene expression six hours after loading, while both the Osteopontin and
the Bcl-2 gene expression showed an increase in gene expression three hours after loading.
Conclusion: These results demonstrate that
real-time RT-PCR is a valuable method for measuring changes in gene expression in bone.
This regimen of mechanical loading induces the expression of Bcl-2, Osteopontin and IGF-1.
The increase in Bcl- 2 gene expression could signify a survival mechanism in osteocytes,
induced by mechanical loading.
[Programme]
P-63
GROWTH FACTORS AS MEDIATORS OF BONE FORMATION AFTER STIMULATION BY
MECHANICAL STRESS
C. Reijnders1*, N. Bravenboer1, J. Hoyland2,
P. Lips1
1Department of Endocrinology, Vrije Universiteit Medical
Center, Amsterdam, The Netherlands
2Musculoskeletal Research Group, University of Manchester
Medical School, Manchester, United Kingdom
Introduction
Mechanical loading and hormones are essential in
maintaining skeletal integrity. Growth factors have a key role in this process. In this
study we developed an in situ hybridisation (ISH) method in order to localize the
mRNA expression patterns of growth factors in rat bone.
Animals and Methods
Tibiae of 12-week-old female Wistar rats were
dissected, fixed and embedded in paraffin. As controls sections of human fractures, human
kidney and rat brain were used. The following ISH techniques were performed:
non-radioactive riboprobe, radioactive riboprobe, in situ reverse transcriptase
polymerase chain reaction (IS-RT- PCR) and radioactive cDNA probe. The genes of interest
were insulin-like growth factor-I (IGF-I), type-I IGF-receptor (IGF-IR), vitamin-D
receptor (VDR) and estrogen receptor alpha (ERalpha).
Results
Both the non-radioactive and the radioactive
riboprobe ISH resulted in a clear positive mRNA expression signal. The brain showed IGF-I
mRNA expression in Purkinje cells of the cerebellum and in neurons of the medulla
oblongata. In the tibiae IGF-I is expressed in osteoblasts, chondrocytes and bone marrow
cells. No IGF-I mRNA expression was detected in osteocytes.
The kidney showed VDR mRNA expression in tubuli and
glomeruli. In the tibiae VDR is expressed in osteoblasts, bone marrow cells and some
chondrocytes, but not in osteocytes.
The IS-RT-PCR method, which was only performed on kidney
sections, also showed VDR mRNA expression in tubuli and glomeruli. However, the morphology
of these structures was dramatically decreased.
The radioactive cDNA ISH showed that ERalpha is expressed
in osteoprogenitor cells, osteoblasts and some osteoclasts in human fractures. In the
tibiae ERalpha mRNA expression was detected in osteoblasts. Unfortunately, the mRNA
expression signals of the IGF-I and IGF-IR cDNA probe were too low to define the
localization.
Conclusions
Both the non-radioactive as well as the radioactive
riboprobe ISH technique resulted in a high mRNA expression signal in combination with a
low background in bone tissue (high sensitivity). Furthermore the positive control tissues
showed their specific gene-expression localization as described by others.
Therefore, this ISH technique is a useful method to
detect acute changes of growth factor mRNA expression levels in bone after stimulation by
mechanical stress.
[Programme]
P-64
OESTROGEN SAVES OSTEOCYTES FROM OXIDANT INDUCED DEATH VIA A RECEPTOR
INDEPENDENT MECHANISM
V. Mann*, C. Towell, G. Kogianni, B. Noble
Muskuloskeletal Research Unit, University of Edinburgh, UK
Evidence exists concerning the anti-oxidant properties of
oestrogen in protecting neuronal cells from oxidative stress. The withdrawal of oestrogen
after menopause is the major factor determining age related bone loss and apoptotic death
of osteocytes. While oestrogen replacement demonstrates clear oestrogen receptor mediated
benefits to bone cells little is known regarding oestrogens' anti-oxidant effects in bone.
Here we have used MLO-Y4 osteocyte-like cell line to
determine whether oestrogen saving effects on osteocytes involves its activities as an
anti-oxidant.
MLO-Y4 cells were treated with physiological doses (10-8)M
of either 17-beta E2 or the oestrogen receptor inactive stereoisomer 17-alpha E2
with or without the specific oestrogen receptor antagonist ICI 182,780 prior to the
addition of 0.4milliM 30% (v/v) H2O2. Cellular apoptosis was
determined using morphological and biochemical criteria.
H2O2 induced an increase in
apoptosis of MLO-Y4 (14.3 ±3 SD vs control 1.4 ±0.9). Pre-treatment of the cells with
17-beta E2 significantly reduced H2O2 induced apoptosis
(2.4 ±0.96). Pre-treatment of cells with 17-alpha E2 or ICI 182,780 also
reduced oxidant induced apoptosis to 3.4 ±1.5 SD and 7.0 ±2.3 respectively.
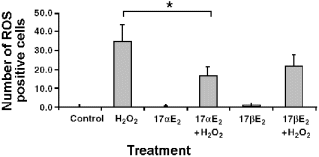
The cellular production of reactive oxygen species was
determined using the free radical indicator 2'7'- dichlorodihydrofluorescein diacetate. H2O2
induced increases in the number of ROS positive cells (34.6 ±9.07 SD vs control 0.22
±0.39 SD). In contrast pre-treatment with both 17-beta E2 and 17-alpha E2
reduced the number of ROS positive cells associated with H2O2
treatment (Fig 1).
These data suggest that oestrogens ability to save
osteocytes from oxidant induced death is independent of the oestrogen receptor and may be
related to oestrogens known activity as an anti-oxidant. This raises the possibility that
loss of osteocytes during oestrogen insufficiency may occur through a failure to suppress
the activity of naturally occurring or disease associated production of oxidant molecules.
[Programme]
P-65
THE ROLE OF FAS/CD95 IN THE GLUCOCORTICOID INDUCED LOSS OF BONE CELLS
G. Kogianni1*, V. Mann1, M. Nuttall2, B.
Noble1
1Muskuloskeletal Research Unit, University of Edinburgh, UK
2Department of Muskuloskeletal Diseases, GlaxoSmithKline, King
of Prussia, USA
Glucocorticoids are successfully used in the management
of a wide variety of diseases but have clear detrimental effects on bone cells indicating
the need to design pharmacological moderators to restore the balance between their
negative and positive effects. Dexamethasone (Dex) induces apoptosis as part of its
immunosuppressive action in a number of different cell types through activation of the Fas
Ligand/Fas Receptor pathway. FasR oligomerisation leads to recruitment of FADD and
activation of caspase-8, which further activates caspases-3 and -7. Several studies have
also reported an association between Fas and ERK pathways in the induction of apoptosis.
The present study investigates the apoptotic pathways activated during Dex-induced death
of osteocyte cultures using a range of techniques.
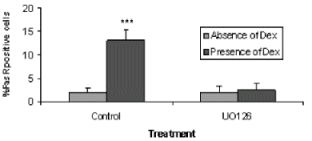
RT-PCR studies revealed that MLO-Y4 osteocytes expressed
FasR both in basal state and following treatment with Dex. Immunocytochemistry revealed
that Dex at 10-6M increased the proportion of cells with surface expression of
FasR by 7-fold (Fig1), with an associated induction of apoptosis (22.8 ±4.1 SD vs control
1.6 ±1.5 SD). Pre-incubation of osteocytes with caspase inhibitors of the Fas pathway
reduced osteocyte apoptosis to 3.0 ±1.1 SD.
Western blot analysis indicated that Dex increased ERK1/2
protein activation which coincided with MEK1/2 and p90rsk kinase activation. In addition
immunohistochemistry revealed that pre-treatment with UO126 inhibitor decreased the number
of cells with membrane expression of FasR, by 4-fold and the proportion of apoptotic
osteocytes to 5.8 ±1 SD.
These findings suggest that Dex activates a caspase
dependent Fas/CD95 apoptotic pathway in osteocytes. In addition Dex induced a rapid
activation of ERK1/2 without which both Fas expression and apoptotic death were reduced.
These data suggest that
ERK might co-operate with Fas in glucocorticoid induced
osteocyte loss and point to possible pharmaceutical intervention in the treatment of
patients receiving glucocorticoids.
[Programme]
P-66
SUBCELLULAR LOCALISATION OF THE THIOREDOXIN/THIOREDOXIN-REDUCTASE-SYSTEM
IN OSTEOBLASTS AND MESENCHYMAL STEM CELLS
K. Paunescu1*, R. Ebert1, D. Schneider1,
K. Becker-Brandenburg2, M. Kassem3, F. Jakob1
1Osteologiezentrum Experimentelle und klinische Orthopädie,
Universität Würzburg, Würzburg, Deutschland
2Interdisziplinäres Forschungszentrum, Universität Giessen,
Giessen, Deutschland
3Department of Endocrinology and Metabolism, Aarhus
Amtssygehus, University Hospital of Aarhus, Aarhus, Denmark
Mesenchymal stem cells (MSC) are a potential source for
cell-based therapies in regenerative medicine, e.g. tissue engineering. During ex vivo
procedures the stem cell genome has to be protected from oxidative damage. The
thioredoxin/thioredoxin- reductase (Trx-TrxR) system is one of several systems active in
antioxidative defense. The selenoprotein TrxR 1 was primarily characterized as a cytosolic
enzyme but, like its substrate Trx, was recently shown to be localized in the nucleus in
kidney cells using immunohistochemistry. Trx is transferred into the nucleus upon
UV-irradiation and H 2O2 and TNF-signalling. The aim of our work is
to investigate the subcellular localisation and nuclear interaction partners of Trx and
TrxR and isoforms of the latter in osteoblasts and mesenchymal precursors.
TrxR cDNA comprises two putative start codons at
positions 97 and 253, the latter of which was so far believed to be the real start codon.
However RTPCR using isoform specific oligonucleotide primers yielded products for both
ATGs, indicating the existence of both mRNA species. TERT 4, HFOBs, T/C-28a2 and HEK 293
cells showed variable amounts of both mRNAs and of TrxR activity. Transient transfection
of GFP fusion proteins (Trx-N1-EGFP, TrxR-N1-EGFP, TrxR-pDsRed2N1) into osteoblast cells
(hFOB) revealed cytosolic localisation of both isoforms. While the isoform ATG1 was also
nuclear, ATG2 was very rarely found in the nucleus. Transfection of the ATG1 to ATG2
fragment alone showed cytosolic and nuclear localisation accordingly. Staining of HFOBs
and mesenchymal stem cells with Trx antibody revealed that Trx was preferentially
localised in the nucleus; using an antibody to TrxR it was shown that the enzyme was
always colocalized with Trx in mesenchymal stem cells, osteoblast-like cells and
chondrocyte like cells. In summary we could characterise the subcellular localisation of
the Trx/TrxR system in osteoblasts and mesenchymal stem cells with respect to the
expression of TrxR isoforms. The role of the ribonucleotide reductase TrxR in the nucleus
remains to be elucidated. Besides its well characterized function in modulation of
transcription factor DNA binding, the role in nuclear antioxidative defense and/or DNA
processing and repair might be hypothesized.
This work was supported by the Deutsche
Forschungsgemeinschaft GK 639.
[Programme]
P-67
HYPERTHERMIA AFFECTS HUMAN OSTEOSARCOMA CELLS
K. Trieb*, H. Blahovec
Dept. of Orthopedics, University of Vienna, Austria
Temperature is a physical factor with strong influence on
growth processes in general and hyperthermia is used as adjuvant therapy in treatment of
cancer patients. In this study, the direct effect of temperature to the osteosarcoma
derived cell lines HOS85, MG-63 and SaOS-2 is investigated. Heat shock was applied by
incubation of one group of cells for 1 hour at degrees C, whereas a second group of cells
was incubated at 37 °C, serving as a control. Alkaline-phosphatase activity was
determined by p-nitrophenyl-phosphate and cell proliferation by tritium labelled
thymidine. Western Blot Analysis were analyzed with a monoclonal antibody specifically
recognizing HSP 70/72. Exposure to 42 °C for 1 hour inhibits proliferation in all three
cell lines. A sublethal heat shock at 42 °C inhibits proliferation, asessed by
3H-thymidine-uptake, most notably in MG-63 to less than 60% compared to cells grown
continuously at 37 °C. Inhibition of proliferation to 63% occurs in HOS85 cells, while in
SaOS-2 cells proliferation only falls to 92% of the control at physiological temperature.
Furthermore a sublethal heat shock decreases alkaline phosphatase activity, the very
marker for osteoblast like cells, in all of the three cell lines. When compared to cells
grown continuously at 37 °C, in cells, which have undergone hyperthermia treatment, a
significant decrease of alkaline phosphatase activity to less than 15% and one third in
HOS85 and SaOS-2, respectively, was found. In MG-63 cells alkaline phosphatase is
decreased to about 75%. In western blot analysis HSP70 was expressed constitutively in
HOS85 cells and was found to be up-regulated in cells exposed to 42 °C for 1 hour. During
recovery of heat shock the expression of HSP70 was decreased to about 50% after 6 hours.
However, after 36 hours the level of expression had been increased to 154% compared to
cells lacking history of hyperthermia. The results of this study indicates that heat shock
has an inhibitory effect on osteosarcoma cells. These data suggest that hyperthermia has
an anti-tumor effect and might be a possible treatment of osteosarcoma.
[Programme]
P-68
HEPATITIS-C VIRUS INFECTION INVOLVES OSTEOBLAST PROGENITORS AND
OSTEOBLASTS IN HEPATITIS-C VIRUS CHRONIC CARRIERS
R. Kluger1*, O. Hoffmann2, H. Mühlberger3,
A. Kröner1, A. Engel1, B. G. Pavlova3
1Department of Orthopaedics; SMZOst Donauspital;
Langobardenstrasse 122 A-1220 Vienna, Austria
2Department of Pharmakology and Toxicology; University of
Vienna; Althanstrasse 14; A-1090 Vienna, Austria
3L.Boltzmann Institute for Leukemia Research and Haematology;
Heinrich- Collinstrasse 30; A-1140 Vienna, Austria
Sterilization of allogenic bone transplants is an
important safety requirement for the reduction of Hepatitis C virus (HCV) transmission
especially during the antibody negative window period. Although it has been shown using
experimental contamination of bone with model viruses that HCV can be effectively
eliminated, there are recent findings demonstrating that HCV replication occurs in CD34+
stem cells. This potentially leads to contamination of osteoprogenitors and osteoblasts
with HCV RNA. To determine whether early bone cell progenitors express HCV RNA, we tested
trabecular bone samples from 6 patients positive for HCV antibody and PCR and 6 negative
patients obtained during hip joint replacement surgery. Stromal cells were incubated with
mouse monoclonal anti-STRO-1, specific for osteoprogenitors followed by incubation with
magnetic beads. STRO-1-positive, osteoprogenitor cells, were then cultured in the presence
of dexamethasone, beta-glycerophosphate, and ascorbic acid for up to 4 weeks to generate
mature osteoblasts, which were than evaluated for alkaline phosphatase, osteocalcin and
osteopontin to confirm maturation. Osteoprogenitors and osteoblasts were tested for the
presence of HCV RNA using strand specific HCV RT PCR and PCR driven in situ hybridization.
Resulting amplicons were sequenced to verify HCV genome. We observed high virus loads in
fresh osteoprogenitors from all HCV positive patients. However, negative strand HCV RNA
was positive in fresh osteoprogenitor cells from 3 of the 6 HCV positive patients.
Remarkably, HCV negative strand RNA was not detected in osteoblasts and in situ PCR
detected intracellular virus present in osteoblasts in 3 out of 6 patients. Osteoblast and
osteoprogenitor cells from negative patients were HCV negative. Taken together, these
results illustrate that HCV positive osteoprogenitor cells and mature osteoblasts are
present in HCV carriers. These data suggest that sterilization procedures be tested for
their ability to eradicate intracellular HCV from allogenic bone grafts.
[Programme]
P-69
VGLUT1 REGULATES DIFFERENTIATION-DEPENDENT VESICULAR GLUTAMATE RELEASE IN
OSTEOBLASTS
P. G. Genever*, G. J. Spencer
Biomedical Tissue Research, Department of Biology, University of York,
York, UK
Evidence has accumulated to suggest that skeletal cells,
including osteoblasts, and osteoclasts, express functional glutamate receptors. This has
drawn comparisons with synaptic signalling in the central nervous system (CNS) where
glutamate acts as an excitatory amino acid. However questions remain over the source of
glutamate in bone, the mechanism of its release and the physiological relevance of fast
excitatory 'neurotransmission' in skeletal tissue.
VGLUT1 and VGLUT2 were recently identified as
'brain-specific' markers of glutamate-releasing neurons in the CNS. The function of VGLUT1
and 2 is to load glutamate into recycling intracellular vesicles. Glutamate is
subsequently discharged into the synapse by fusion of the vesicle membrane with the plasma
membrane. We have exploited these advances to clarify the mechanism and function of
glutamate exocytosis in bone.
Immunoreactivity for VGLUT1 and VGLUT2 was identified in
periosteal cells and, more prominently, in differentiated osteoblasts at endosteal and
periosteal bone surfaces of neonatal rat tibia. Using a specific fluorimetric assay, we
demonstrated that human bone marrow stromal cells (BMSCs) released glutamate. The level of
glutamate release increased significantly (+50%) during osteogenic differentiation of
BMSCs and decreased (-30%) following adipogenic induction, corresponding with alterations
in VGLUT1 mRNA and protein expression profiles. Application of exogenous glutamate to
BMSCs increased viable cell numbers by up to 40% over 7 days using strictly controlled
culture conditions, whereas pharmacological inhibiton
of glutamate release induced apoptosis. Real-time optical
determinations using live osteoblastic cells demonstrated that glutamate exocytosis and
vesicle recycling were temporally coordinated, sustained and slow compared to the rapid
release activity of depolarised neurons. In MG-63 osteoblastic cells, VGLUT1 and VGLUT2
immuno- colocalised with FM1-43 (a tracer of actively recycling vesicles) in perinuclear
pools and at peripheral cytoplasmic sites. Identical distribution patterns were observed
in MG-63 cells transfected with VGLUT1 fused to enhanced green fluorescent protein and
overexpression of VGLUT1 in MG-63 cells increased the proportion of total cellular
glutamate secreted from 30% (in mock-transfected cells) to 65%, 72 hours
post-transfection.
These findings demonstrate that continuous vesicular
glutamate exocytosis operates in osteoblasts through differentiation-dependent VGLUT1
expression, which may provide a sophisticated mechanism for regulating viable osteoblast
numbers at remodelling sites.
[Programme]
P-70
THE RELATIONSHIP BETWEEN RANKL EXPRESSION AND OSTEOBLAST DIFFERENTIATION
IN HUMAN OSTEOBLASTS
G. J. Atkins1*, P. Kostakis1,2, B. Pan2,
A. N. Farrugia2, S. Gronthos2, A. Evdokiou1, D. M.
Findlay1, A. C. W. Zannettino2
1Dept. of Orthopaedics and Trauma, University of Adelaide,
Adelaide, South Australia, Australia
2Division of Haematology, Institute of Medical and Veterinary
Science, Adelaide, South Australia, Australia
Cells of the osteoblast lineage support two apparently
distinct functions, bone formation and promotion of osteoclast formation. The aim of this
study was to examine the relationship between these phenotypes in human osteoblasts
(NHBC), in terms of the pre-osteoblast marker, STRO-1 and the mature osteoblast marker,
alkaline phosphatase (AP), and the expression of genes involved in osteoclast formation,
RANKL and OPG. The osteotropic stimuli, 1alpha,25(OH)2vitaminD3 (vitD3) and
dexamethasone, were found to have profound proliferative and phenotypic effects on NHBC.
VitD3 inhibited NHBC proliferation and increased and maintained the percentage of cells
expressing STRO-1 over an 18 day culture period, implying that vitD3 promotes and
maintains the proportion of cells displaying an immature osteogenic phenotype.
Concomitantly, RANKL mRNA expression was up- regulated and maintained in NHBC in response
to vitD3. Messenger RNA encoding M-CSF, an essential co-factor for osteoclast
differentiation, was coordinately expressed with RANKL mRNA in these cells. Culture for
longer periods with vitD3 in the presence of beta-glycerophosphate resulted in effective in
vitro mineralisation by NHBC. Dexamethasone progressively promoted the proliferation
of AP-expressing cells, resulting in the overall rapid maturation of the cultures.
Dexamethasone had little effect on RANKL mRNA expression and down-regulated OPG mRNA
expression in a donor-dependent manner. Regression analysis showed that RANKL mRNA
expression was associated negatively with the percentage of cells expressing AP (p <
0.01), in vitD3 and dexamethasone treated NHBC. In contrast, RANKL mRNA expression was
associated positively with the percentage of STRO-1-positive cells (p < 0.01). In NHBC
sorted by FACS on the basis of STRO-1 expression (STRO- 1bright and STRO-1dim
populations), it was found that vitD3 up-regulated the expression of RANKL mRNA
preferentially in STRO-1bright cells. The results suggest that immature
osteoblasts respond to vitD3 in a potentially pro-osteoclastogenic manner by the
coordinated expression of M-CSF and RANKL mRNA, and this response is sustained by an
increase in the osteoprogenitor pool. They suggest also, that the dual roles of
osteoblasts, in supporting osteoclastogenesis or forming bone, may be performed by the
same lineage of cells at different stages of their maturation, providing a potential
explanation for the obligatory linkage between these two processes.
[Programme]
P-71
THE USE OF STEREO VISUALIZATION IN A BMU-BASED SIMULATOR FOR BONE
REMODELLING
R. Phillips1*, J-A. Grunchec1, J. W. Ward1,
M. J. Fagan2, C. A. Dobson2, C. Langton3, G. Sisias4
1Department of Computer Science, University of Hull, Hull, UK
2Department of Engineering, University of Hull, Hull, UK
3Centre of Metabolic Bone Disease, University of Hull, Hull,
UK
4Department of Computing, University of Bradford, Bradford, UK
Software simulators for bone remodelling provide insights
into the bone remodelling process, the emulation of bone diseases and the effect of
treatments for these diseases. Visualization in 3D of bone structures further helps bone
researchers to understand the complex geometry of bone, how bone changes over time and the
structural characteristics of bone. This visual understanding is enhanced by using
stereoscopic visualization as it improves depth perception of trabeculae.
We have developed a simulator for the bone remodelling
process of trabecular structure of cancellous bone where bone is modeled by voxels whose
sides are 20 microns. BMUs (Basic Multi-cellular Units) are modeled by simulating the net
effect of osteoclasts and osteoblasts at activation sites. The simulation is controlled by
various user defined formulae. For example, formulae define the probability that voxels
will be activated and the extent of the activation. These formulae may contain variables
such as location, bone age and bone strain. As remodelling changes strain in the bone our
simulator recalculates the new pattern of strain of bone voxels using a finite element
technique.
The simulator provides various monoscopic and
stereoscopic views of simulated bone remodelling. These views are created using a
volumetric rendering technique based on a texture map technique. We are able to view bones
structures in stereo on PC workstation monitors and on our large active stereo workwall (5
x 2.5 metres). The user can interrupt a remodelling simulation at will and then fly around
the bone structure whilst viewing it in stereo. In addition the surface can be coloured to
show the pattern of bone strain (or age). Animations may also be produced in stereo that
show how the bone changes during the simulation. These various stereo views and animations
allow bone researchers to understand such issues as a) how and why the shape of trabeculae
change, b) where and when perforations occur in bone structures, c) the relationships
between strain and age and remodelling, etc. We have used the bone remodelling simulator
to investigate normal remodelling behaviour and also to investigate bone diseases, such as
osteoporosis and rheumatoid arthritis and treatments.
[Programme]
P-72
GLUCOCORTICOID RECEPTORS ARE MODULATED BY GP130- MEDIATED CYTOKINES IN
THE HUMAN OSTEOBLAST-LIKE CELL LINES SAOS-2 AND MG-63: EFFECTS OF INTERLEUKIN-11,
ONCOSTATIN M AND LEUKEMIA INHIBITORY FACTOR
A. Dovio1*, M. L. Sartori1, B. Ceoloni1,
L. Saba1, S. Racca2, A. Angeli1
1Internal Medicine Unit, Department of Clinical and Biological
Sciences, University of Turin, Italy
2Pharmacology, Department of Clinical and Biological Sciences,
University of Turin, Italy
The gp130 family of cytokines includes interleukin
(IL)-6, IL-11, oncostatin M (OSM), leukemia inhibitory factor (LIF), ciliary neurotrophic
factor, cardiotrophin-1 e B-cell stimulating factor-3. They share similarities in
structure and the commun signal-transducing receptor subunit gp130. Most gp130 cytokines
and their receptors are expressed in the bone microenvironment, and are credited with
osteoclastogenic activity in vitro, mainly through up-regulation of the expression of
RANK-L by cells of the osteoblastic lineage. Moreover, gp130 cytokines stimulate
osteoblast differentiation of mesenchymal precursors. We have previously demonstrated that
IL- 6 is an autocrine-paracrine positive modulator of glucocorticoid (GC) receptors (GR)
in the human osteosarcoma cell lines Saos-2 and MG-63. GR is a key determinant of tissue
sensitivity to GC. The aim of the present study was to extend our investigation to other
osteotropic gp130 cytokines, i.e. IL-11, LIF and OSM. Cells were incubated with the
cytokines (IL-11: 0.1-100 ng/ml; LIF and OSM: 0.05-50 ng/ml) for 20 h; afterwards, GR
number and affinity were determined by radioligand binding assay. In MG-63 cells both
IL-11 and OSM dose-dependently decreased GR number, while LIF was ineffective; data from
radioligand binding assay were confirmed by Western blot analysis with a specific
anti-GRalpha antibody. In Saos-2 cells autocrine down- regulation of GR by IL-11 was shown
using a specific anti-human IL-11 antibody. None of the three cytokines consistently
modified GR affinity. The divergent effects of IL-11 with respect to IL-6 could be
explained by mutual regulation of secretion. In order to investigate this hypothesis, we
have measured IL-6 and IL-11 levels in the supernatants of cells treated with IL-11 and
IL-6, respectively. No reciprocal regulation between IL-6 and IL-11 was observed. On the
contrary, OSM was found to stimulate both IL-6 and IL-11 release. Since GC are known to
modulate cytokines secretion and activity, and cytokines are able, in turn, to modulate GR
number, our results are consistent with the notion of autocrine-paracrine modulatory loops
of GC sensitivity. They add to the recently demonstrated regulation by inflammatory
cytokines of 11beta-hydroxysteroid dehydrogenase activity. They could be of relevance in
the pathogenesis of the biphasic bone loss observed in patients treated with GC who have
immune-mediated diseases and conceivably high concentrations of gp130 cytokines in the
bone microenvironment.
[Programme]
P-73
DEXAMETHASONE AND BMP-2 INDUCE A MORE DIFFERENTIATED OSTEOBLAST PHENOTYPE
IN HUMAN BONE MARROW DERIVED STROMAL CELLS
Z. Henriksen*, O. H. Sørensen, N. R. Jørgensen
The Osteoporosis and Bone Metabolic Unit, Dept. of Endocrinology and
Clinical Biochemistry, Copenhagen University Hospital, H:S Hvidovre, Denmark
In vitro models of bone cells are important for the study
of bone biology including the regulation of bone formation and resorption. For the study
of osteoblasts in vitro, both cell lines and primary cultures have been used. In this
study we compared two primary culture systems based on stromal cells obtained from human
bone marrow. Osteoblast phenotypes were induced by either dexamethasone (Dex) or bone
morphogenetic protein-2 (BMP-2).
Bone marrow was obtained from biopsies at the posterior
iliac spine from young healthy volunteers, aged 20-31. Cells were isolated by gradient
centrifugation and grown to confluence, approximately 6 weeks. Cells were treated with
100nM Dex and/or 100ng/ml BMP-2 for the last 7 days before experiment (three weeks in the
mineralization assay). The osteoblast phenotype was
assessed as alkaline phosphatase (AP) activity/staining, production of osteocalcin and
pro-collagen type 1 (P1NP), parathyroid hormone (PTH) induced cyclic AMP (cAMP) production
and in vitro mineralization.
AP activity was increased by Dex treatment, but not by
BMP-2 treatment. P1NP production was significantly decreased after Dex treatment, while
BMP-2 had no effect on P1NP levels. Osteocalcin production was low in cultures not
stimulated with 1,25-dihydroxyvitamin D. Dex or BMP-2 treatment alone did not affect the
basic osteocalcin levels, but in combination with 1,25-dihydroxyvitamin D, BMP-2 increased
the osteocalcin production, while Dex treatment completely suppressed osteocalcin
production. Further PTH induced cAMP production was greatly enhanced by Dex treatment,
whereas BMP-2 did not affect cAMP production. Finally, in vitro mineralization was greatly
enhanced in cultures enriched with BMP-2 and, to a lesser extent, Dex. Cell proliferation
was only increased significantly by Dex treatment.
In conclusion, both models described produce cells with
an osteoblastic phenotype. Though cells from both systems can produce mineralized matrix,
the effect on bone proteins are clearly different. This could imply that, to a certain
degree, the production of bone matrix proteins and mineralization could be regulated
independently.
[Programme]
P-74
VEGF (VASCULAR ENDOTHELIAL GROWTH FACTOR) IN OSTEOPOROSIS IN-VIVO AND
IN-VITRO
T. Pufe1*, K. Scholz-Ahrens2, A. T. M. Franke1,
W. Petersen3, D. Varoga1, R. Mentlein1, B. Tillmann1,
J. Schrezenmeir2, C. C. Glueer4
1Department of Anatomy, Christian-Albrechts-University Kiel,
Germany
2Institute of Physiology and Biochemistry of Nutrition
3Department of Orthopaedic Surgery
4Medical Physics, Dept. Diagn. Radiol
Objective: Osteoporosis is a disease characterized by low
bone mass and an increased susceptibility to fractures. The molecular mechanism of GC
induced osteoporosis and the occurrence and function of related cytokines are largely
unclear. Aim of this investigation was to analyse the influence of glucocorticoid (GC)
treatment on the VEGF expression in-vitro and in an animal model (Göttinger Minipig).
Methods: As part of a larger study, 17 primiparous sows
were allocated to 2 experimental groups (control and GC treated animals). Human
osteoblasts were used for in vitro assays. VEGF and VEGF receptors were located by immuno-
histochemistry. VEGF content in lumbar vertebra and culture supernatants were measured by
enzyme-linked immunosorbent assay (ELISA); VEGF mRNA was detected by reverse
transcription-polymerase chain reaction (RT-PCR). Spinal bone mineral density (BMD) was
assessed in vivo by Quantitative Computed Tomography (QCT).
Results: Strong VEGF concentrations were measured in
normal lumbar vertebrae whereas VEGF concentrations were 60 % lower (p<0.0001) in
GC-treated minipigs. Our in-vitro experiments with cultured osteoblasts confirmed the
in-vivo findings. Osteoblasts were immunopositive for VEGF. VEGF receptors VEGFR-2 (KDR,
flk- 1) and VEGFR-1 (flt-1) could be immunostained on osteoclasts and osteoblasts. VEGF
mRNA and protein was detectable in all lumbar vertebrae. Incubation with dexamethasone
decreased VEGF secretion also in vitro to 39 % (p<0.01).
BMD assessed by QCT at study start was comparable for the
control group and the GC treated group. Over the 15 month duration of the study BMD was
stable in the control group (n=9) (-0.46 ±2.2 percent, n.s.) but decreased in the GC
group (n=8) by -2.8 ±5.5 percent. The difference in the rate of loss was highly
significant (p<0.0014). VEGF levels were significantly correlated with this change in
BMD (r=0.7).
Conclusion: VEGF is produced in osteoblasts and its
concentration is decreased in GC treated animals as well as in osteoblasts exposed to GC.
Since reductions in VEGF concentrations correlate with parallel measurement of bone
mineral density in GC treated minipigs and VEGF is as angiogenesis factor suitable for
recruiting basic multicellular units (BMU) we hypothesize that VEGF may be an important
modulating factor for bone remodeling, specifically in GC induced osteoporosis.
[Programme]
P-75
THE ADMINISTRATION OF EP4 SELECTIVE AGONIST ACCELERATES CORTICAL BONE
HEALING AFTER DRILL-HOLE INJURY IN RATS
M. Tanaka1*, A. Sakai1, S. Tanaka1, S.
Uchida1, M. Nagashima1, T. Katayama2, K. Yamaguchi2,
T. Nakamura1
1Department of Orthopaedic Surgery, University of Occupational
and Environmental Health, Kitakyushu, Japan
2Fukui Safety Reseach Institute, Ono Pharmaceutical Company,
Osaka, Japan
Prostaglandin E2 (PGE2) stimulates bone formation and
increases bone mass when dosed systemically or locally. Of the four PGE2 receptor subtypes
(EP1-EP4), EP4 is considered to be the major receptor that mediates an anabolic effect on
bone. This investigation was performed to clarify the hypothesis that EP4 selective
agonist accelerates cortical bone healing in drill-hole injuring rats. A total of 128 male
Wistar 12-week-old rats were studied. In this model, a hole measuring approximately 2.0 mm
in diameter penetrating the bone marrow was drilled in the anterior portion of the
diaphysis of bilateral femurs. Rats were injected subcutaneously with vehicle or two doses
(10ug/kg, and 30ug/kg body weight) of EP4 selective agonist (ONO-4819 CD) twice a day. We
started the administration on the next day of the surgery and continued it until a day
before killing. At days 0, 5, 7, 14, 21, and 28 after surgery, the injured rats (n=8 in
each group) were sacrificed. The injured sites of the femurs were analyzed using
peripheral quantitative computed tomography (pQCT), bone histomorphometry, and
biomechanical testing in three point bending. The results of pQCT showed that in the EP4
agonist-administered rats, cortical bone mineral content and cortical bone mineral area
increased significantly and dose-dependently at day 21 compared with those in the
vehicle-administered control rats. Histomorphometric analysis showed that in the EP4
agonist-administered rats, the cortical bone volume (BV/TV) increased significantly and
dose-dependently at days 14 and 21 compared with that in the control rats. The values of
ultimate load in the EP4 agonist- administered rats increased dose-dependently. The values
of bone metabolic markers, urinary deoxypyridinoline and serum osteocalcin, in the EP4
agonist-administered rats did not differ significantly from those in the control rats.
Bone mineral density (BMC) of the forth lumbar body in the EP4 agonist-administered rats
did not differ from that in the control rats. We concluded that the administration of EP4
selective agonist accelerates cortical bone healing after drill hole injury in rats. The
EP4 agonist at the doses used in this experiment did not affect the systemic bone
turnover, but the injured sites selectively.
[Programme]
P-76
CYTOSKELETAL REORGANIZATION STIMULATES THE OSTEOBLASTIC DIFFERENTIATION
C. Higuchi1,2*, K. Yoshioka1, H. Yoshikawa2,
K. Itoh1
1Department of Biology, Osaka Medical Center for Cancer and
Cardiovascular Diseases, Osaka, Japan
2Department of Orthopedic Surgery, Osaka University Medical
School, Suita, Osaka, Japan
Cytoskeletal change appears to be one of the cellular
signalings, which modify the cellular differentiation. To prove this hypothesis, we herein
focused on the effects of actin cytoskeletal change on the osteoblastic differentiation.
Employing two actin polymerization-interfering reagents, cytochalacin D and latrunculin B,
we examined morphological change, actin polymerization status, alkaline phosphatase (ALP)
activity, osteocalcin (OCN) secretion, and mineralization of extracellular matrix in mouse
preosteoblastic cell line MC3T3-E1 in the absence or presence of recombinant human bone
morphogenetic protein-2 (rhBMP-2). Long lasting treatment of the cells with these reagents
did not affect ALP activity, but inhibited cell proliferation in their high concentration.
In contrast, one-hour treatment with these reagents promoted the osteoblastic
differentiation including the increase in ALP activity, OCN secretion, and mineralized
nodule formation without inhibiting cell proliferation. Actin stress fibers and focal
adhesions in the cells once disappeared and reappeared following reorganized within 1 hour
after the treatment. Moreover, to investigate the relationship between the osteoblastic
differentiation and cytoskeletal change, we examined ALP activity by the stimulation with
rhBMP-2 before or after the cytoskeletal reorganization. The increase in ALP activity by
the treatment with these reagents diminished and disappeared when the cytoskeletal
reorganization was completed in the cells. In addition, Y27632, a specific inhibitor for
Rho kinase, which has been reported to affect actin stress fiber formation, had the same
positive effects on the osteoblastic differentiation. Our data indicated that transient
actin cytoskeletal reorganization was a novel positive cellular signaling for the
osteoblastic differentiation.
[Programme]
P-77
EVIDENCE THAT VASCULAR ENDOTHELIAL GROWTH FACTOR AND PROSTAGLANDIN E2
SIGNALLING COUPLES OSTEOBLAST AND ENDOTHELIAL CELL BEHAVIOUR
C. E. Clarkin*, A. A. Pitsillides, C. P. D. Wheeler-Jones
Department of Veterinary Basic Sciences, Royal Veterinary College,
London, UK
A continuous vascular supply is crucial during
development, remodelling and repair of bone, yet little is known of the mechanisms by
which the relationship between endothelial and bone cells is regulated and coordinated.
The purpose of this study was to evaluate the potential roles of Vascular Endothelial
Growth Factor (VEGF) and prostaglandin E2(PGE2) as autocrine/
paracrine signalling molecules by investigating their effects on primary osteoblasts (OBs)
and endothelial cells (ECs). In human umbilical vein ECs (HUVEC), VEGF165(25
ng/ml) induced early and robust ERK1/2 activation, late COX-2 protein induction, and the
release of prostanoids (PGI2 and PGE2). In contrast, human and
rat-derived OBs showed little ERK1/2 activation or COX-2 induction in response to VEGF165/164(1-100
ng/ml), but were, however, capable of some VEGF-induced prostanoid release. While it is
established that VEGF is mitogenic for ECs, we observed little effect of exogenous VEGF164on
OB proliferation. This is consistent with an apparent lack of VEGFR2 (Flk-1) protein
expression in OBs, which was clearly evident in HUVEC by immunoblotting. This differential
sensitivity to VEGF was not apparent in the responses to exogenous PGE2; thus,
both HUVEC and OBs exhibited enhanced ERK1/2 phosphorylation, COX-2 induction and PGI2
synthesis following challenge with 1 uM PGE2. These
results suggest that: i) in bone, VEGF is likely to
preferentially target endothelial cells and, ii) since PGE2 is thought to
stimulate osteoblast VEGF production (Harada et al., J Clin Invest 93, 2490, 1994 ) it is
also likely that this osteoblast-derived VEGF exerts a selective paracrine action on
endothelial cells. This would ensure continued PGE2 production and provide the
basis for an autoamplification mechanism that tightly couples the behaviour of these two
cell types.
[Programme]
P-78
GENERATION OF HUMAN OSTEOBLAST-LIKE CELLS FROM UMBILICAL CORD BLOOD
E. L. Hutson1*, S. Boyer2, P. G. Genever1
1University of York, Biomedical Tissue Research, Heslington,
York, UK
2Smith & Nephew GRC, York, UK
Identifying appropriate cellular sources for
transplantation therapies and tissue engineering is a primary research goal in
regenerative biology. Umbilical cord blood (UCB) is routinely used to extract
haematopoietic stem cells expressing the cell surface marker CD34. However, there is
growing evidence to suggest that stem cells with the ability to generate
non-haematopoietic cell types may also exist in UCB. Mesenchymal stem cells (MSCs) are
found predominantly in bone marrow and are able to differentiate into osteogenic,
chondrogenic and tenogenic lineages, offering potential for generating autologous tissue
transplants. However, the acquisition of MSCs from bone marrow is invasive and
restrictive. We have now identified conditions that will routinely generate mesenchymal
cells from UCB, which are able to undergo osteogenic differentiation.
Blood was extracted from the cord and placenta following
pre-term caesarian sections, via informed consent, and separated through Ficoll prior to
culture in adherent conditions for 0-9 days. We demonstrated by flow cytometric analysis
that expression of the haematopoietic lineage markers CD2, CD19, CD38 and CD66b decreased
significantly over this time period; expression of CD14 (monocyte/macrophage marker) was
maintained; whilst an increase in expression of the mesenchymal markers, CD29, CD44, CD105
and CD166 was observed. Immunomagnetic depletion of cells expressing CD34 and CD14 from
the initial UCB cultures prevented the accumulation of monocyte/macrophage-like cells and
promoted the growth of adherent stromal cells. When cultured under osteogenic conditions
for up to 30 days, small numbers of these cells formed distinct colonies with
morphological characteristics of osteoblasts. These osteoblast-like cells expressed
alkaline phosphatase, osteopontin, osteonectin, osteocalcin, Cbfa1, type I collagen and
formed von Kossa-positive bone nodules in vitro. Adhesion, proliferation and osteogenic
differentiation of the stromal cell population from UCB were enhanced by growth on
fibronectin and collagen, compared to laminin and plastic substrates. Our studies have
also demonstrated that UCB-derived stromal cells have the potential to incorporate into
biomimetic scaffolds in a manner similar to MSCs. These findings suggest that blood may
act as a source of progenitor cells that are able to undergo osteogenic differentiation
under defined conditions, which will significantly impact on the development of autologous
tissue engineered bone constructs.
[Programme]
P-79
TYPE I COLLAGEN SYNTHESIS BY HUMAN OSTEOBLASTS IN RESPONSE TO PLACENTAL
LACTOGEN AND CHAPERONIN 10, A HOMOLOGUE OF EARLY PREGNANCY FACTOR
J. P. Mansell*, S. J. Yarram, N. L. Brown, J. R. Sandy
Division of Child Dental Health, University of Bristol, Bristol, UK
Recent studies have indicated that maternal skeletal
metabolism undergoes significant changes during gestation (Naylor et al. 2000 & Black
et al. 2000). The agents that are responsible for eliciting these changes in bone turnover
during pregnancy have yet to be defined. We therefore sought to investigate whether
chaperonin 10 (Cpn10), a homologue of early pregnancy factor (Fletcher et al. 2001), or
human placental lactogen (PL), were capable of influencing the synthesis of type I
collagen by primary human osteoblasts in vitro. Both Cpn10 and PL are major components of
the maternal circulation during pregnancy but how they might contribute to bone metabolism
has not been determined. Type I collagen represents the most abundant component of bone
tissue, accounting for approximately 90% of the organic compartment. Both Cpn10 and PL
were capable of stimulating the synthesis of type I collagen by human osteoblasts in
culture by 70 and 250% of controls respectively. The levels of the house keeping
gelatinase, MMP-2, were also raised following treatment with Cpn10 or PL whereas alkaline
phosphatase activity and cell proliferation were unaffected. The inclusion of
17b-oestradiol failed to influence any of the parameters tested. These novel findings
support a role for PL and Cpn10 in the metabolism of bone tissue during pregnancy.
Maternal bone collagen metabolism is clearly an important event during pregnancy and the
identification of the factors responsible will aid our understanding of the regulation of
skeletal metabolism during gestation.
References:
Black et al. 2000. JBMR 15; 557-563.
Fletcher et al. 2001. Mamm. Genome. 12; 133-140.
Naylor et al. 2000. JBMR 15; 129-137.
[Programme]
P-80
EXPRESSION OF CALCITONIN- AND CALCITONIN RECEPTOR- LIKE RECEPTOR IN HUMAN
OSTEOBLAST-LIKE CELLS
I. Villa1*, E. Mrak1, A. Rubinacci1, F.
Guidobono2
1Bone Metabolic Unit, Scientific Institute H San Raffaele,
Milano, Italy
2Dept. of Pharmacology, Chemotherapy and Medical Toxicology,
University of Milano, Milano, Italy
Calcitonin (CT), calcitonin gene-related peptide (CGRP)
and amylin belong to the same family of peptides, the genes for which have a common
ancestral origin. The three peptides share structural homology and produce similar
biological effects in many tissues including bone. Each peptide of the CT- gene family
binds to its own specific receptor. However, the three peptides are also able to
cross-react with each other's receptor. Some of them have been cloned: the CT receptor
(CTR) and the CT receptor-like receptor (CRLR). Their affinity for each peptide depends on
the relative expression of accessory proteins or receptor activity-modifying proteins
(RAMPs), which constitute a group of three proteins designated as RAMP1, 2 and 3. The
expression of these receptors in human osteoblast is still controversial. The present
study was designed to assess the possible expression of CRLR, CTR and RAMPs in human
osteoblast-like cells (hOB). The expression of hCRLR, hCTR, hRAMP1-3 was evaluated by
RT-PCR in primary culture of hOB cells obtained from bone explants deriving from different
donors. Preliminary RT-PCR results show that hOB cells express the genes for the CTR, CRLR
and RAMP1, whereas, so far, RAMP2 and RAMP3 do not appear to be expressed.
The physiological role of the receptors for the peptides
of the CT family on osteoblast is not known. However, this finding together with the
already demonstrated proliferative effects of CGRP and CT on hOB ( Villa et al., Am J
Physiol, in press ) could encourage the development of new molecules with anabolic action
on osteoblasts that could be useful for the therapy of osteoporosis particularly for the
senile one that involves osteoblast senescence.
[Programme]
P-81
A NOVEL ANTI-RHEUMATIC DRUG, T-614, STIMULATES OSTEOBLASTIC
DIFFERENTIATION IN VITRO AND BONE MORPHOGENETIC PROTEIN-2 INDUCED BONE FORMATION IN VIVO
BY INCREASED THE EXPRESSION OF OSTERIX
K. Kuriyama1,2*, C. Higuchi1,2, K. Tanaka3,
H. Yoshikawa1, K. Itoh2
1Department of Orthopaedic Surgery, University of Osaka,
Suita, Japan
2Department of Biology, Osaka Medical Center for Cancer and
Cardiovascular Disease, Osaka, Japan
3Research Laboratories, Toyama Chemical Co., Ltd., Toyama,
Japan
T-614 (N-[3-(formylamino)-4-oxo-6-phenoxy-4H-chromen-7-yl] methanesulfon-
amide), a newly developed anti-rheumatic drug under clinical trial, is an anti-
inflammatory agents presenting the inhibitory effect of bone destruction in vivo arthritis
model. We found that T-614 stimulated osteoblastic differentiation of stromal cell line
(ST2) and preosteoblastic cell line (MC3T3-E1) in the presence or absence of recombinant
human bone morphogenetic protein-2 (rhBMP-2). Calcium content of mineralized nodules was
14-fold elevated by the addition of T-614 in the presence of rhBMP-2 in ST2 but not
MC3T3-E1. Oral administration of T-614 to mice also promoted rhBMP-2 induced bone
formation in vivo. We also examined the phosphorylation levels of Smad 5 and the
expression levels of Id1 direct targets of BMP signaling. T-614 did not affect the
phosphorylation levels of Smad 5 nor the expression levels of Id1. The transcription
factors, Cbfa1/Runx2 and osterix, both play essential roles in osteoblast differentiation.
The expression levels of Cbfa1/Runx2 were not stimulated by T-614, while the
transcriptional levels of osterix were 3-fold increased by T-614 with rhBMP-2 in ST2.
Together, we speculated that T-614 was not involved in the early signal transduction
pathway of BMP-2 but a stimulator of osterix. Therefore, T-614 presented novel anabolic
effects on bone metabolism, besides suppressor of bone resorption, by increased the
expression of osterix, and good therapeutic potential for the treatment of osteoporosis.
[Programme]
P-82
CBFA1 EXPRESSION IN THE DIFFERENTIATION OF BONE MARROW-DERIVED
OSTEOBLASTS
Q. Qu*, X. H. Wan, C. Z. Chen
Medical Research Center, Peking Union Medical Collage Hospital, Beijing,
China
Cbfa1 is a transcription factor recognized as the
osteogenic master gene in early osteoblast differentiation. Since dexamethasone (Dex)
induces osteoblast differentiation from its bone marrow precursors and enhances mature
osteoblast phenotype, we examined Cbfa1 expression pattern in the presence or absence of
Dex using a mouse bone marrow culture system. The bone marrow was harvested from the shaft
of mouse femurs. At day 4, confluent cells in primary cultures were trypsinized and
subcultured. The cultures with Dex showed sequential and higher expression levels of
osteoblastic phenotype markers type I collagen, alkaline phosphatase, and osteocalcin
during a three-week culture period. Matrix mineralization observed by von Kossa staining
was also more evident in Dex-treated cultures. RT-PCR was used to study the expression of
Cbfa1 and glyceraldehyde-3-phosphatase dehydrogenase
(GAPDH) at different time points. GAPDH expression was
similar in control and in Dex-treated cultures, demonstrating similar starting quantities
of RNA from each sample. Cbfa1 mRNA was initially detected in primary cultures from day 3
and the highest expression level was observed on day 6 of subcultures. The levels of Cbfa1
mRNA expression were constant in cultures with or without Dex at all time points examined.
By immunofluorescence examination, we found that Cbfa1 staining started to appear on day 5
of primary cultures and co-localized with cell nuclear. Such staining was not obvious
after day 13 of subcultures. The appearance of Cbfa1 staining was similar in cultures with
or without Dex. In conclusion, we show that Cbfa1 expression was associated with a
characteristic manner during the differentiation of osteoblasts in bone marrow cultures.
The osteogenic-inducing effect of Dex may be independent of Cbfa1 function.
[Programme]
P-83
MODULATION OF OSTEOBLASTIC CELLS ACTIVITY ON IMPROVED TITANIUM CARBIDE
(TIC) COATED SUBSTRATUM
A. Ricci1*, M. Brama1, D. Ferro2, G. De
Maria2, S. Migliaccio4, R. Teghil3, L. Politi1,
R. Scandurra1
1Depts of Biochemistry, University of Rome, La Sapienza, Italy
2Centro di Termodinamica Chimica alle Alte Temperature, CNR,
Roma
3University of Basilicata, Potenza
4Medical Pathophysiology, University 'La Sapienza', Italy
The biocompatibility of orthopedic or dental implants
depends on the effect of the implant on bone-forming cells, osteoblasts. Stable connection
between biomaterial surface and surrounding tissue is one of the most important
prerequisites for the long- term success of implants. Therefore, a strong adhesion of the
cells on surface is required. Titanium is the most largely used material in dental and
orthopedic implants due to suitable physical properties and good biocompatibility.
However, its integration into bone, which is mainly mechanical in nature, may be poor and
responsible for clinical failure. To improve the integration between implant and bone
tissue, our aim was to develop a new more biocompatible form of titanium and to
characterize potential effects on osteoblasts homeostasis. Titanium(Ti) samples were
coated with a layer of its carbide (TiC), producing the double effect of protecting the
metal against its oxidation and making the metal harder. Next step was to evaluate whether
TiC would modulate osteoblastic cells activity in vitro. ROS.SMER#14 osteoblast-like cells
were plated on Ti samples or a layer of TiC deposited by Pulsed Laser Deposition
technology (PLD) and characterized by surface roughness. Cells were grown for different
times (6, 12, 24 hr) and then evaluated by Scanning Electron Microscopy (SEM), which
showed that cells plated on TiC spreaded and attached firmly to the substrate faster than
cells plated on Ti, suggesting that TiC might improve osteoblastic cells adhesion.
Further, to evaluate potential modulation of bone specific markers, cells were plated and
grown o.n. Total RNA was extracted, reverse transcribed in cDNAs, amplified by PCR using
proper primers and products resolved by electrophoresis and quantified by densitometry.
Genes involved in bone turnover, as collagen1A2, osteopontin, osteocalcin, BMP-4 and
alkaline phosphatase, were all increased in cells grown on TiC as compared to Ti.
Moreover, cells grown on TiC showed lower expression of IL-6 and M-CSF, suggesting that
osteoclasts activity might also be reduced by a paracrine mechanism.
In conclusion, our data suggest that TiC is a valid
material to coat orthopedic and dental prostheses, since improves osteoblasts adhesion and
activity, thus improving bone cells homeostasis at the implant site.
[Programme]
P-84
EXPRESSION OF ANTIMICROBIAL PEPTIDES IN BONE
D. Varoga1*, T. Pufe1, W. Petersen2, B.
Tillmann1, O. Kloppenburg3, F. Paulsen1
1Dept. of Anatomy, University of Kiel, Kiel, Germany
2Dept. of Orthopaedic Surgery, University of Kiel, Kiel,
Germany
3Dept. of Orthopaedic Surgery, Ostseeklinik Damp, Damp,
Germany
Introduction: The innate immune system represents an
ancient host defense mechanism. The most important effector mechanism of cell-mediated
innate immunity is the production of antimicrobial peptides (AP) in response to pathogens.
The upregulation of the inducable antimicrobial peptides is mediated by Toll-like-
receptors (TLR). The purpose of the study was to determine whether human bone express
antimicrobial peptides and toll-like-receptors under regular conditions and to investigate
potential differences in case of inflammatory bone disease. Moreover we examined the AP-
and TLR- expression on cultivated osteoblast cells (HOB) in stimulation experiments.
Materials and Methods: Healthy and inflamed bone was
obtained from the Department of orthopaedic surgery. After removal the samples were
prepared for immunohistochemistry and RT-PCR. Quantification of the AP-mRNA was done with
Real-Time RT-PCR. The cultivated human osteoblast cells (HOB) were used for stimulation
experiments. The alteration of the AP-expression in presence of proinflammatory cytokines
or bacteria was assessed by immunohostochemistry, RT- PCR and Real-Time RT-PCR.
Results: Immunohistochemistry and RT-PCR revealed
different antimicrobial peptides in healthy bone and the osteoblast culture. The
expression pattern of the AP changed in case of inflammatory bone disease. The stimulation
experiments revealed the increase of the inducable beta defensins 2 and 3 in presence of
LPS or proinflammatory cytokines measured by Real-time RT-PCR. Toll-like-receptors, needed
for the induction of the AP, were detected by RT-PCR on the cultivated osteoblasts.
Conclusion: The human bone produces a variety of
antimicrobial peptides. Under inflammatory conditions the expression pattern of the
antimicrobial peptides changes. This response seems to be mediated by toll-like receptors
(TLR) which were present on cultured osteoblasts. Synthetic HBD-3 shows antimicrobial
effects even to multiresistant Staph. aureus in-vitro, so it may be useful in the
treatment of inflammatory bone disease in future. The role of the antimicrobial peptides
in inflammatory bone disease awaits further elucidation.
[Programme]
P-85
ROLIPRAM POTENTIATES PARATHYROID HORMONE-MEDIATED CELL SURVIVAL IN MG-63
OSTEOSARCOMA CELLS
M. M. Huttunen*, M. Pekkinen, M. E. B. Ahlström, C. J. E.
Lamberg-Allardt
Division of Nutrition, Department of Applied Chemistry and Microbiology,
University of Helsinki, Helsinki, Finland
Parathyroid hormone (PTH) has been shown to protect
osteoblasts and osteosarcoma cells from apoptosis (Jilka et al. 1999). PTH inhibitory
effect on osteoblast apoptosis is mediated by cAMP (Jilka et al. 1999, Turner et al.
2000). Concentration of intracellular cAMP-molecules falls as a result of
phosphodiesterase (PDE) activity (Conti et al. 1995, Ahlström & Lamberg-Allardt
1997). We speculated that inhibition of phosphodiesterase (PDE) activity could potentiate
the protective effects of PTH. Using selective PDE-inhibitors, anion-exchange
chromatography and RT-PCR we identified the phosphodiesterase profile of human MG-63
osteosarcoma cells. The main PDE activities found in this cell line was shown to consist
of PDE1 (20%), PDE4 (67%) and rolipram/IBMX-insensitive PDE (25%). The PDE isoforms 4A and
4B but no 4C or 4D were identified by RT-PCR. The cells contained no measurable PDE2 or
PDE3 activity. Apoptosis was induced by challenging the cells with 50 nM etoposide for six
hours. Etoposide caused statistically significant (P<0,05) increase in cell death.
Using two concentrations of PTH (10 nM and 1 nM) we investigated the effect of the PDE4
selective PDE-inhibitor rolipram on cell survival. The 10 nM dose of PTH inhibited
etoposide induced cell death significantly whereas the 1 nM PTH nor 32 microM rolipram
alone had no effect. However, combinations of 1 nM PTH and 32 microM rolipram caused
statistically significant increase in cell survival. Our results show that the PTH dose
required to protect MG-63 cells from undergoing apoptosis can be considerably lowered by
inhibition of PDE4 by rolipram.
[Programme]
P-86
MECHANICAL REGULATION OF HB-GAM EXPRESSION IN OSTEOBLAST-LIKE CELLS
A. M. Liedert*, P. Augat, L. Claes
Institute of Orthopaedic Research and Biomechanics, University of Ulm,
Ulm, Germany
Heparin-Binding Growth-Associated Molecule (HB-GAM),
orginally isolated from rat brain, was also abundantly found in bovine bone tissue.
Studies from Imai et al. (1998) with transgenic mice overexpressing HB-GAM demonstrated a
role of HB- GAM in promoting bone formation. However, little is known about the role of
HB- GAM in the process of bone formation, e.g. proliferation and differentiation.
The purpose of this study was to investigate the
mechanisms of mechanoreception and signal transduction involved in the regulation of
HB-GAM expression by mechanical stimulation. Monolayer cultures of SaOs-2 cells on
silicone dishes were subjected to cyclic, homogenous stretching in a longitudinal
direction by four-point- bending. An amplitude of 1000 microstrain, 1800 cycles, and a
frequency of 1 Hz was applied. Specific inhibitors of signal transduction pathways were
added to the culture medium before loading and control incubation. RT-PCRs were performed
with specific primers for HB-GAM and GAPDH as control.
We could show that mechanical stimulation of SaOs-2 cells
resulted in a rapid decrease (32 %, p < 0.05) of HB-GAM expression directly after
completion of loading. Studies with RGD peptides demonstrated that the interaction of
integrines with matrix molecules containing the RGD sequence is involved in the mechanical
regulation of HB-GAM expression. Experiments with cytochalasin D showed that the integrity
of the cytoskeleton is necessary for downregulation of HB-GAM expression. By using the ion
channel blockers gadolinium III-chloride and nifedipine we could demonstrate that Ca2+
channels of the stretch-activated type (SA-cat type) and of the voltage-sensitive type
(L-type) are involved as mediators of the mechanical signal, too. Treatment of the
cultures with 18-a-glycyrrhetinic acid showed that the cell-to- cell communication by gap
junctions plays an important role in the mechanism of downregulation of HB-GAM expression.
Together these findings suggest that multiple mechanisms
of the bone cell are responsible for uptake and transmission of mechanical stimulus
signals inducing downregulation of HB-GAM expression in bone cells.
[Programme]
P-87
LOW AND HIGH CONCENTRATIONS OF HEPARIN HAVE DIFFERENT EFFECTS ON
OSTEOBLAST-LIKE SAOS-2 CELLS IN VITRO
H. Hausser*, R. Brenner
Division for Biochemistry of Joint and Connective Tissue Diseases,
University of Ulm, Ulm, Germany
Long term treatment with heparin has been associated with
an increased risk of osteoporosis. Given the well known importance of heparan sulfate
proteoglycans for bone metabolism, it can be anticipated that heparin due to its
structural similarity with heparan sulfate chains somehow interferes with the biological
activities of these cell surface- and extracellular matrix-associated molecules. In order
to shed some light on the effect(s) of heparin on osteoblasts possibly contributing to the
development of heparin-induced osteoporosis, we treated osteoblast-like Saos-2 cells in
monolayer culture for different periods of time with different concentrations of heparin.
During the initial proliferative phase heparin led to a significant increase in cell
number as judged by mitochondrial dehydrogenase activity, clearly indicating that heparin
at least does not inhibit the proliferation of Saos-2 cells. After longer incubation
times, however, in cells treated with higher concentrations of heparin (5 microg/ml), a
decrease in cell number could be observed that was not obvious in cells treated with low
heparin concentrations (5 - 500 ng/ml). Surprisingly, these low heparin concentrations
promoted the deposition of a collagenous matrix and its subsequent mineralization, whereas
concentrations of heparin 5 microg/ml clearly inhibited mineralization. In these latter
cultures a dramatic loss of cells occurred upon addition of 10 mM beta-glycerophosphate as
a phosphate donor to induce mineralization. Positive TUNEL staining indicated that this
loss of cells was at least in part due to apoptosis. Apparently, high concentrations of
heparin sensitize Saos-2 cells to phosphate-induced apoptosis, whereas low concentrations
of heparin promote matrix deposition and mineralization. Thus, as is the case in other
systems, the effects of heparin on osteoblast-like cells appear to be biphasic. Most
importantly, our results imply that low dose heparin might even prove to be beneficial for
bone formation.
[Programme]
P-88
ADHESION SIGNAL ACTIVATION OF OSTEOBLASTIC CELLS TO RECOMBINANT HUMAN
OSTEOPONTIN FRAGMENTS CONTAINING DIFFERENT CELL BINDING MOTIFS
Y-J. Lee*, J. S. Ko, H-M. Kim
College of Dentistry, Seoul National University, Seoul, Korea
Osteopontin (OPN) is one of major extracellular matrix
proteins rich in bone and one of the adhesion ligands where osteoblasts bind through
integrin adhesion receptors. OPN which is highly expressed in osteoblasts has three major
cell binding motifs within a relatively short sequence such as ELVTDFPTDLPAT (aa131-143),
RGD (aa159-161), SVVYGLR (aa162-168). Among these sequences, SVVYGLR is known to be
exposed only when thrombin cut OPN into two fragments between 168 and 169. In the present
study, osteoblastic cell responses to various osteopontin fragments of different cell
binding motifs were examined using recombinant fusion human osteopontin fragments. Full
rhOPN containing three cell binding motifs, rhOPN17-168 containing three cell binding
motifs without a C-terminal sequence, rhOPN17-164 with two cell binding motifs, or
rhOPN17-150 with one cell binding motif were produced as fusion proteins with
six-histidine tag in E.coli. Culture surfaces were pre-coated with different rhOPN
fragment and allowed HOS osteoblastic cells to attach without serum. rhOPN17-150 was
lowest in activating adhesion signals such as focal adhesion kinase, paxillin, and
extracellular signal- regulated kinase 1/2 as well as in cellular adhesion and
proliferation. rhOPN17-168 followed rhOPN17-150 in the activation of adhesion signals.
rhOPN17-164 and full rhOPN showed the highest activation of adhesion signals in a similar
way. These results indicate that RGD motif in OPN is a major cell binding motif for
osteoblast adhesion which may be exposed when OPN is intact as a full molucule and other
two motifs of SVVYGLR and ELVTDFPTDLPAT may contribute as a minor role to osteoblast
adhesion to OPN.
[Programme]
P-89
ENDOTHELIN-1 EFFECTS ON OSTEOBLASTIC DIFFERENTIATION DEPEND ON THE LEVEL
OF CONNEXIN43 EXPRESSION
C. Niger*, M. Mesnil, L. Cronier
CNRS UMR6558, University of Poitiers, Poitiers, France
Gap junctional intercellular communication (GJIC) permits
coordinated cellular activities during development and differentiation processes, and its
dysfunction or mutations of gap junction protein (connexin, Cx) genes have been implicated
in human pathologies. In bone, in vitro studies have demonstrated the implication
of Cx43 expression and GJIC in osteoblastic differentiation and mineralization of the
extracellular matrix. Recently, it was demonstrated that Cx43 knock out mice exhibited
developmental defects of the craniofacial skeleton and impairment of osteoblastic
differentiation, supporting the fact that Cx43 expression and GJIC are of critical
importance for normal bone formation. Endothelin-1 (ET1) has been also implicated in the
control of osteoblastic proliferation and differentiation. Indeed, ET1 knock out mice
revealed the involvement of this peptide in proliferation and migration of osteogenic
cells and an inhibitory action of ET1 was shown on bone mineralization. However, although
ET1 is a uncoupling agent and decreased GJIC have been implicated in carcinogenesis, no
data are available on the ET1 action on GJIC and Cx43 expression in osteoblastic cells. In
the present study, a possible cross talk between Cx43 and ET1 was tested in
temperature-sensitive human cells (hFOB1.19) which display a proliferative or
differentiated phenotype when cultured at 33.5 °C or 39 °C respectively. Interestingly,
Cx43 protein expression, GJIC and alkaline phosphatase (ALP) activity were significantly
reduced in proliferative hFOB1.19 cultured at 33.5 °C compared to 39 °C. The perifusion
of ET1(10-8M) in the vicinity of cultured osteoblastic cells induced a rise of
intracellular calcium activity ([Ca2+]i) confirming the presence of ET1 receptors in the
two phenotypes. However, ET1 caused a peak of [Ca2+]i followed by a lasting plateau in
proliferative cells, while on differentiated cells ET1 only induced a slow and lasting
increase. Moreover, the mitogenic effect of ET1 was significantly higher in osteoblastic
cells cultured at 33.5 °C as measured by MTT assay or growth rate determination.
Furthermore, a differential ET1 effect was demonstrated on the ALP activity.
In conclusion, these preliminary data strongly suggest
that the level of Cx43 expression influences the osteoblastic response to endothelin-1.
This work was partly supported by The Ligue Régionale
contre le cancer.
[Programme]
P-90
BONE CELLS FROM OSTEOARTHRITIC AND OSTEOPOROTIC DONORS DIFFER IN THEIR
RESPONSE TO MECHANICAL LOADING IN VITRO
A. D. Bakker1*, H. F. Teshale1, E. Tanck2,
I. C. Heyligers3, G. H. Albers4, P. Lips5, E. H.
Burger1, J. Klein-Nulend1
1Dept. of Oral Cell Biology, ACTA-Vrije Universiteit,
Amsterdam, The Netherlands
2Orthopaedic Research Laboratory, University of Nijmegen,
Nijmegen, The Netherlands
3Dept. of Orthopaedics, Vrije Universiteit Medical Center,
Amsterdam, The Netherlands
4Dept. of Orthopaedics, Hospital Hilversum, Hilversum, The
Netherlands
5Dept. of Endocrinology, Vrije Universiteit Medical Center,
Amsterdam, The Netherlands
Osteoporosis is a condition characterized by loss of bone
mass, while osteoarthritis is associated with increased bone mass. Bone mass is determined
by mechanical stimuli, but also by the response of osteocytes to loading-induced flow of
fluid through the lacuno-canalicular network. The aim of this study was to compare bone
cells derived from osteoporotic (OP) and osteoarthritic (OA) donors in their response to
mechanical loading, in the form of a pulsating fluid flow in vitro.
Bone cells from 9 female OP donors (age 60-90 year) and 9
age and gender- matched OA donors were subjected to mechanical loading using pulsating
fluid flow (PFF) of low shear stress (0.4±0.2 Pa at 3 Hz), high shear stress (0.6±0.3 Pa
at 5 Hz), or were kept under static control conditions. After 1 h PFF at 5 Hz, cells were
post- incubated for 24 h without PFF. Nitric oxide (NO) and prostaglandin E2
(PGE2) production, as well as COX2 mRNA, were determined as parameter of bone
cell responsiveness.
Mechanical loading by means of PFF at 3 Hz increased NO
production by OP, but not OA bone cells. PFF at 5 Hz resulted in a greater stimulation of
NO production in OP than in OA cells (OP 5.4-fold; OA 2.8-fold increase). PFF at 3 Hz did
not provoke a PGE2 response, but PFF at 5 Hz significantly increased PGE2
production by 2 to 3- fold in both OP and OA cells. Although one hour of PFF significantly
stimulated COX2 mRNA expression by 3.5-fold in both OP and OA bone cells, the consequent
PGE2 production during the 24 h post incubation period was significantly
stimulated in OA cells, but not in OP cells.
In conclusion, this study found differences in the
response of bone cells from osteoporotic and arthritic donors to mechanical stress, as
indicated by the NO and long-term PGE2 response to fluid flow. The altered
capacity of bone cells to respond to mechanical stress might be a cause of abnormal bone
metabolism in OP and OA patients.
[Programme]
P-91
INTERLEUKINE (IL)-8 AND IL-18 SERUM LEVELS ARE STILL ELEVATED IN
ENDOGENOUS CUSHING SYNDROME FOLLOWING TREATMENT DESPITE SIGNIFICANT IMPROVMENTS IN BONE
METABOLISM
C. Kristo1,2*, K. Godang1, T. Ueland1,2,
R. Jemtland1, P. Aukrust3, J. Bollerslev1
1Section f Endocrinology, National University Hospital, N-0027
Oslo, Norway
2Research Center for Internal Medicine
3Section of Clinical Immunology and Infectious Diseases
Patients with endogenous Cushing Syndrome (CS) have
elevated cortisol production most often because of a tumor in the pituitary gland or
adrenal cortex. These patients also have decreased bone mass and enhanced risk for
osteoporotic fractures. In glucocorticoid induced osteoporosis (GIO) bone resorption is
increased, but paradoxically, bone formation is reduced.
Increased apoptosis of mature osteoblasts and osteocytes
may be one of the mechanisms of GIO uncoupling.
Immunological mediators have been shown to influence bone
metabolism. Accordingly, we recently found significantly elevated serum levels of the
proinflammatory interleukins (IL)-8 and IL-18 in 33 untreated CS patients.
The aim of the present study was to measure serum levels
of IL-8 and IL-18 in CS following treatment. Moreover, to investigate the potential
relation of the cytokines to alterations in osteodesitometry and biochemical markers of
bone metabolism.
Material: 25 patients with CS (21 patients with pituitary
adenoma, 4 with adrenal tumor, 17 women, 8 men) were examined following operative
treatment, mean follow- up 31 months (range 5-80 months). The patients were compared to
age, -sex, and BMI- matched controls. The controls were re-examined mean 50 months after
the first examination (range 26-56 months).
Methods: Cytokine levels and biochemical markers of
bone-turnover were analyzed by immuno-assays, and bone mass was measured by Dual-Energy
X-ray Absorptiometry (DEXA).
Results: Our main findings in CS patients after treatment
were: (i) BMD and bone mineral content (BMC) in the lumbar spine (LS) and the femoral neck
(FN) increased significantly in CS patients. (ii) In CS patients serum levels of
osteocalcin increased significantly, whereas cross-laps increased, but not significantly.
(iii) IL-8 decreased significantly but not to levels found in healthy controls. (iv) IL-18
continued to increase but not significantly compared to baseline.
Conclusions: The present study demonstrates that patients
with CS following treatment continue to have elevated serum levels of IL-8 and IL-18
although biochemical markers of bone turnover increase and bone mass improves. Serum
levels of cytokines may not necessarily reflect the cytokine levels in the bone
microenvironment. However, our findings suggest that immunopathogenic mechanisms may be
operating in CS and related to the disturbed bone homeostasis.
[Programme]
P-92
HYDROCORTISONE INCREASES THE RATE OF DIFFERENTIATION OF CULTURED PRIMARY
HUMAN OSTEOBLASTS
D. C. Ireland*, S. Bord, J. E. Compston
University of Cambridge School of Clinical Medicine, Addenbrooke's
Hospital, Box 157, Cambridge, UK
Supraphysiological glucocorticoid concentrations can
cause osteoporosis. A dose- dependent increase in risk of fragility fractures is seen
three months after therapy is started but risk declines rapidly when medication is
stopped. Histomorphometry of bone biopsies from glucocorticoid-treated patients has shown
reduction in cancellous bone formation. However, the mechanisms involved are not clearly
understood. A decrease in osteoblast replication and differentiation and an increase in
mature osteoblast apoptosis have been suggested
We hypothesized that supraphysiological hydrocortisone
concentrations increase, not decrease, human osteoblast (hOB) differentiation and that
ALP/COL1 ratios increase more rapidly in cells cultured with increased hydrocortisone
concentrations. We cultured sub-confluent young and old donor hOBs from different
anatomical sites in McCoy's medium supplemented with 100micromolar long-life ascorbic
acid, 10percent human serum, 200millimolar glutamine and increasing doses of
hydrocortisone. On day zero, 4micromolar beta-cyclodextrin was added to two flasks of
control cells and 0.2micromolar (in normal adult physiological range) and 4micromolar
(typical plasma level after oral glucocorticoid administration) cyclodextrin-encapsulated
hydrocortisone were added to two flasks each. The medium was changed every two days. One
flask of cells at each hydrocortisone concentration was harvested at two days and the
remaining flasks were harvested at 4 days (young donor hOBs (hand)) or 8 days. Poly(A) RNA
was extracted and ALP and COL1 mRNA levels were determined based on comparative
threshold-cycles in real-time fluorescence-based RT-PCR.
Changes in ALP/COL1 mRNA ratios during the culture period
were calculated. Results indicate dose-dependent increase in hOB differentiation with
increased hydrocortisone concentration. The data suggest a model in which glucocorticoid-
induced enhanced osteoblast differentiation reduces bone formation by limiting the amount
of collagen synthesized before maturation of the extracellular matrix allows
mineralization.
Changes in ALP/COL1 mRNA ratios with time in human
osteoblasts. Values are x- fold for day 8 (or day 4) relative to day 2. Those marked *
were statistically significant p<0.05.
|
added hydrocortisone concentration |
hOB line |
0micromolar |
0.2micromolar |
4micromolar |
young donor (hand) |
-1.02 |
+1.99* |
+2.93* |
young donor |
-2.33* |
+1.79* |
not done |
old donor (hip) |
-1.61* |
-1.16 |
+1.49* |
old donor (neck) |
-4.55* |
-1.49* |
+1.06 |
[Programme]
P-93
THE AAP ANALOGUE ZP123 INCREASES INTERCELLULAR COUPLING IN HUMAN
OSTEOBLASTS AND STIMULATES PRODUCTION OF BONE FORMATION MARKERS IN VITRO
S. S. Hansen1*, Z. Henriksen1, J. S. Petersen2,
O. H. Sørensen1, N. R. Jørgensen1
1The Osteoporosis and Bone Metabolic Unit, Dept.s of
Endocrinology and Clinical Biochemistry, Copenhagen University Hospital, H:S Hvidovre,
Denmark
2Zealand Pharma A/S, Glostrup, Denmark
Signaling via gap junction channels is a way for
osteoblastic cells to communicate and synchronize activities. This signaling is essential
for bone cells in order to express proteins specific for bone formation and
mineralization. Antiarrhytmic peptide (AAP) is an endogenous peptide that increases gap
junctional coupling in cardiomyocytes. ZP123 is a new AAP analogue that in contrast to AAP
is stable in enzymatic environment. To examine acute and long-term in vitro effects
of AAP stimulation, human bone marrow derived osteoblastic (hOB) cells were treated with
ZP123 for either ten minutes (acute) or four weeks (long-term). hOB cells were obtained
from human bone marrow by puncture of the posterior iliac spine of young, healthy
volunteers, aged 20-35. Cells were cultured with ZP123 during normoxia (21 % O2)
or hypoxia (3% O2). Gap junction intercellular communication (GJIC) was
measured by the non-invasive parachute assay and detected using fluorescent activated cell
sorting. Results (mean ±SEM; *: p<0.05, **:p<0.01; ***:p<0.001).
This study shows that both acute and long-term in
vitro treatment with the new stable AAP analogue ZP123 produces a sustained increase
in GJIC in hOB cells.
|
ZP123
nM |
GJIC
(1 month treatment) |
GJIC
(10 minutes treatment) |
| |
|
%Vehicle response |
Normoxia |
0.1 |
145±7*** |
n.d |
| |
10 |
119±5*** |
105±2* |
Hypoxia |
0.1 |
113±5* |
n.d |
| |
10 |
107±6 |
134±16 |
[Programme]
P-94
OSTEOCALCIN IS A MODULATOR OF TRIIODOTHYRONINE (T3) AND
1,25-DIHYDROX-VITAMIN D3 (VITD) REGULATED MMP-13 EXPRESSION IN OSTEOBLASTS
F. Varga*, M. Rumpler, S. Spitzer, K. Klaushofer
Ludwig Boltzmann Institute of Osteology, 4th Medical Dept., Hanusch
Hospital, Vienna, Austria.
T3 and VitD are important regulators of bone development
and metabolism. It was shown that these hormones regulate osteoblastic gene expression.
MMP-13 is one of the most important proteases of the bone tissue. It is expressed by
osteoblasts and hypertrophic chondroblasts and was suggested to prepare the bone matrix
for the osteoclastic resorption, and is possible involved in the regulation of bone
resorption.
Osteocalcin is the most abundant non-collagenous protein
of the bone matrix. Although, the osteocalcin depleted mouse has a higher bone mass and
better bones, the function of this protein is still unknown. Recently, a receptor for
osteocalcin was postulated and it was shown that bovine osteocalcin can modulate the
metabolic activity of osteoblast-like cells. This modulatory effect depends on
pretreatment with hormones.
The MC3T3-E1 cell line is an excellent model system to
study the expression of genes and the differentiation of mouse osteoblasts in vitro.
Several clones with different differentiation states were isolated and characterised. We
studied whether osteocalcin can influence the T3 and VitD regulated expression of MMP-13.
MC3T3-E1 cells (original, clone 4 and 30) were seeded in
alphaMEM supplemented with 5%FCS and treated with VitD and/or T3 and/or bovine osteocalcin
at different concentrations. RNA was isolated and analysed by Northern analysis using
multi-primed labelled cDNA of MMP-13 and osteocalcin. Ribosomal cDNA was used for
normalisation and statistical analyses were performed with ANOVA.
We found that T3 and VitD increased the expression of
MMP-13 and osteocalcin with different amplitudes depending on the MC3T3-E1 clone
investigated, but with an inverse relationship between osteocalcin and MMP-13. Cotreatment
of MC3T3-E1 cells with both hormones suggested a synergistic relationship on the
regulation of MMP-13 with a threshold between 1nM and 10nM VitD at both 10nM and 100nM T3
concentrations. Osteocalcin did not regulate MMP-13 expression but we found that
cotreatment with osteocalcin increased the VitD stimulated MMP-13 expression while it
attenuated the T3 stimulated dose dependently. In conclusion, we present new evidence for
an osteocalcin receptor and show that osteocalcin can modulate the hormonal regulation of
MMP-13 expression in osteoblasts. These data suggest a new role for osteocalcin in bone
metabolism, and demonstrate that this protein is involved in the regulation of gene
expression.
[Programme]
P-95
RESPONSE AND SENSITIVITY OF MURINE OSTEOBLAST- AND OSTEOCYTE-LIKE CELL
LINES TO IONIZING RADIATION
C. E. Hellweg*, P. Lau, S. Kirchner, C. Baumstark-Khan, G. Horneck
Institute of Aerospace Medicine, German Aerospace Center (DLR), Koeln,
Germany
The loss of bone mass is one of the most important
changes which astronauts are subjected to under weightlessness. This decrease in bone mass
is a limiting factor during longterm space missions and has to be restricted by
appropriate countermeasures. Several investigations have disclosed some aspects of
microgravity response of the skeleton and of osteoblasts.
Another important factor in space flight is exposure to
cosmic ionizing radiation. Concerning the bone metabolism, effects of ionizing radiation
and their interaction with microgravity have not been not investigated, especially their
influence on differentiation of different types of bone cells. Related cell types like
fibroblasts show accelerated senescence in response to radiation. In osteoblasts and
osteocytes, similiar terminal differentiation events could be initiated by radiation.
This study focusses on radiation effects on osteoblast-
and osteocyte-like cells. The murine osteocyte-like cell line MLO-Y4 (murine long bone
osteocyte Y4) and the osteoblast-like cell line OCT-1 (both kindly provided by L.
Bonewald, San Antonio, Texas, USA) and two murine embryonic calvaria osteoblast-like cells
MC3T3-E1 subclones with high and low osteoblastic differentiation potential (subclone 4
and 24) were screened for different markers. The growth properties of the four cell lines
were examined, revealing the slowest growth for MC3T3-E1 subclone 4. For these four cell
lines, dose-effect relationships for survival and strand breaks after exposition to X-
rays were determined. Survival was determined in colony forming ability tests or, for
slowly growing cell lines, in a microplate assay. Strand breaks were measured by
fluorescence analysis of DNA unwinding (FADU).
[Programme]
P-96
FUNCTIONAL ROLE OF NOTCH SIGNALLING IN MESENCHYMAL DIFFERENTIATION:
TEMPORAL CONTROL OF OSTEOGENESIS BY GAMMA-SECRETASE
N. A. Matthews1*, T. M. Skerry2, V. Knauper1
1Biomedical Tissue Research, University of York, York, UK
2Department of Veterinary Basic Sciences, Royal Veterinary
College, London, UK
Notch proteins are cell surface receptors critically
involved in cell fate determination. Notch signalling plays a role in regulation of stem
cells in a range of tissues, but its function in osteogenesis is unclear. Ligand
activation of Notch receptors results in presenilin-1 (PS-1)-dependent gamma-secretase
cleavage of Notch within its intracellular domain to release a cytoplasmic fragment (NICD)
that translocates to the nucleus. Interaction of NICD with CSL proteins results in
activation of HES genes, which encode transcription factors that regulate lineage-
specific gene expression.
Using RT-PCR and western blot analysis, we demonstrated
expression of Notch-1 and PS-1 in primary cultures of rat bone marrow stromal cells
(rBMSCs) and MC3T3- E1 osteoblast-like cells. Notch-1 immunoreactivity was identified at
the cell surface, whilst PS-1 was found throughout the cytoplasm and sporadically in
peripheral assemblies. These localisations are consistent with an active signalling
pathway.
To investigate the consequences of impaired Notch
signalling, transition state analogue inhibitors of gamma-secretase were used (DAPT,
WPE-III-31C and JLK7) to treat primary rBMSCs cultured under osteogenic conditions. Using
western blot analysis of whole cell lysates we demonstrated that NICD levels were markedly
decreased in samples treated with DAPT and WPE-III-31C, compared with untreated controls.
In cells incubated with JLK7, there was no difference in NICD expression. Inhibitors of
gamma-secretase all reduced cell numbers, suggesting they reduced proliferation and may
have increased differentiation. This is partially supported by the ability of DAPT and
WPE-III-31C to significantly increase alkaline phosphatase activity compared to controls.
Mineralisation of colonies was reducedin cultures incubated with DAPT and WPE-III-31C,
although addition of JLK7 resulted in a 2- fold increase in both alkaline phospatase
activity and colony mineralisation.
These data suggest that Notch signalling is fundamentally
involved at various stages of osteoblastogenesis. Understanding these complex
differentiation signals could lead to novel therapies for the treatment of bone disorders
such as osteoporosis.
[Programme]
P-97
STRETCH-INDUCED ERK PHOSPHORYLATION DEPENDS ON DIFFERENTIATION STAGE OF
OSTEOBLASTS
J. H. W. Jansen1, F. A. A. Weyts1,2, I. Westbroek1,2*,
H. A. P. Pols2, J. A. N. Verhaar1, H. Weinans1, J. P. T.
M. van Leeuwen2
1Orthopaedic Research Laboratory, Erasmus MC, The Netherlands
2Department of Internal Medicine, Erasmus MC, The Netherlands
Little is known about the mechanisms that enable bone
cells to respond to mechanical stimuli. Mitogen-activated protein kinases (MAPKs) play a
key role in the transfer of many extracellular signals from membrane to nucleus.
Activation of extracellular signal-regulated kinase (ERK, a MAPK subgroup) has been shown
to regulate differentiation of mesenchymal stem cells towards the osteogenic lineage and
to be essential for growth and differentiation of human osteoblastic cells. Recently,
increased phosphorylation of ERK (ERK-P) has been reported in response to mechanical
stretch in ROS cells. In previous experiments we showed the significance of the
differentiation stage of osteoblasts for the translation of hormonal and mechanical
signals. Here, we tested 1: whether human osteoblasts respond to stretch with increases of
ERK-P , and 2: whether this response depends on the differentiation stage of the cells.
The human osteoblast cell line SV-HFO was stretched using
a loading unit (modified Flexcell bioflex instrument [Flexcell Corp., Hillsborough, NC,
USA]) producing strain levels from physiological to supraphysiological after 7, 14, or 21
days of culture. Following stretching, cells were lysed and protein was analysed by
Western blotting using monoclonal anti-ERK-P, or anti-ERK1/2.
SV-HFO cells were cultured in the presence of
dexamethasone and beta- glycerophosphate to induce osteogenic differentiation. Presence of
dexamethasone and beta-glycerophosphate resulted in a peak of alkaline phosphatase
activity and onset of mineralisation at day 14, and mineralisation at 21 days of culture.
Baseline ERK and ERK-P levels gradually increased during osteoblast differentiation. At
all time-points during differentiation stretch didn't change total amount of ERK. Stretch
induced a transient (peak at 5 minutes) increase in ERK-P: 1.5-fold and 2-fold at day 14
and day 21, respectively. In conclusion, this study demonstrates that osteoblasts respond
to stretch by increased ERK-P. The extent of phosphorylation is highly dependent on the
differentiation stage of the osteoblast, i.e. the highest when the extracellular matrix is
mineralized and the osteoblasts become entrapped in the mineralized matrix.
[Programme]
P-98
SUBCELLULAR LOCALISATION OF ENOS AND CAVEOLIN-1 IN CELLS OF THE
OSTEOBLAST LINEAGE
J. MacPhee*, M. Helfrich
Department of Medicine and Therapeutics, University of Aberdeen,
Aberdeen, Scotland
Osteocytes are terminally differentiated osteoblasts that
have become embedded within the bone matrix and play an important role in mechanosensing
and in communicating perceived strain to other bone cells. Osteocytes respond to
mechanical strain by production of a number of signalling molecules, including nitric
oxide (NO). NO is synthesised via NO synthase (NOS), and both endothelial NOS (eNOS) and
neuronal NOS (nNOS) have been reported in osteocytes. Osteocytes rapidly produce NO via
eNOS in response to fluid shear stress. As yet, it is unknown how this rapid response is
achieved, but a similar response has been seen in endothelial cells, where eNOS is
localised specifically in caveolae, characterised by presence of caveolin-1. Within
caveolae eNOS is in close proximity to kinases such as PI-3 kinase and Akt, which can
phosphorylate and thereby activate eNOS. Additionally, certain integrins, such as
alpha5beta1, involved in sensing shear stress, have been shown to localise to caveolae in
endothelial cells, where they may participate in eNOS activation through integrin-linked
kinase and Akt.
To investigate possible mechanisms for eNOS activation,
we examined the subcellular localisation of eNOS, caveolin-1 and beta1 integrins in the
murine osteoblastic cell line MC3T3, primary mouse and human osteoblasts and the murine
osteocytic cell line MLO-Y4. Cells were cultured on collagen-coated glass coverslips,
stained with fluorescently tagged antibodies to eNOS, caveolin-1, and integrin subunits
and examined with a LSM 510 confocal microscope.
Caveolin-1 was membrane associated in all cells and most
strongly expressed in human osteoblasts. eNOS was localised predominantly in the
perinuclear region, rather than in the plasma membrane and most strongly expressed in
primary cells. Integrin subunits alpha5 and beta1 were highly expressed in the plasma
membrane of all cell types, but they were excluded from the caveolae.
In conclusion, in resting osteoblastic cells, eNOS and
alpha5beta1 integrin are not specifically localised in caveolae. Whether there is a rapid
relocalisation to caveolae upon exposure to fluid shear stress, facilitating eNOS
activation, is currently under investigation.
[Programme]
P-99
MODULATION OF HUMAN OSTEOPROGENITOR ACTIVITY USING SYNTHETIC P-15
COLLAGEN BINDING DOMAIN
X. B. Yang1*, H. I. Roach1, N. M. P. Clarke1,
R. S. Bhatnagar2, S. Li3, R. O. C. Oreffo1
1University Orthopaedics, University of Southampton, General
Hospital, Southampton, SO16 6YD, UK
2Laboratory of Connective Tissue Biochemistry, University of
California San Francisco, USA
3Department of Bioengineering, University of California
Berkeley, USA
The generation of biomimetic microenvironments that
exploit extracellular matrix cues for mesenchymal cell differentiation offers tremendous
potential for skeletal regeneration. Type-1 collagen provides a structural framework for
connective tissues and plays a central role in the temporal cascade of events leading to
the formation of new bone from progenitors. A synthetic 15-residue peptide, P-15, related
biologically to the active cell binding domain of type I collagen, has been found to
promote adhesion and osteogenesis of human dermal fibroblasts on particulate anorganic
bone
mineral (ABM). The aim of this study was to examine the
ability of the collagen peptide, P-15, to promote human osteoprogenitor attachment,
proliferation and differentiation on 3-D scaffolds.
Human osteoprogenitors were selected, expanded and
cultured on particulate microporous ABM ('pure' hydroxyapatite) phase and polyglactin
vicryl mesh coupled with or without P-15 in basal (MEM supplemented with 10% FCS) or
osteogenic (MEM/10% FCS supplemented with 10nM dexamethasone and 100uM ascorbate-2-
phosphate) conditions. Cell adhesion, spreading and patterning were examined by
[3H]Thymidine incorporation, light and confocal microscopy following incorporation of cell
tracker green and ethidium homodimer fluorescent labels as well as scanning electron
microscopy (SEM).
Time-lapse photomicroscopy showed increased human
osteoprogenitor attachment, spreading and patterning as well as increased alkaline
phosphatase specific activity. Collagen and osteocalcin expression in basal and osteogenic
cultures compared to control cultures. Extensive cell ingrowth and cellular bridging
between 3-D ABM matrices and polyglactin vicryl mesh coupled with P-15 was observed by
confocal microscopy as well as extensive mineralisation by von-Kossa and alizarin red
staining. In contrast, negligible cell growth was observed on ABM or mesh alone. In vivo
diffusion studies using MF1nu/nu mice showed bone matrix formation and organised collagen
formation as assessed by Sirius red/ alcian blue staining and collagen birefringence after
6 weeks. These in vitro and in vivo studies indicate that P- 15 provides a permissive
environment for osteoprogenitor differentiation along the osteogenic lineage.
In summary, these experiments indicate the potential of
P-15 to generate appropriate biomimetic microenvironments to promote osteoprogenitor
differentiation and demonstrate the potential for the exploitation of extracellular matrix
cues for osteogenesis and their application to bone tissue engineering.
[Programme]
P-100
HUMAN OSTEOPROGENITOR BONE FORMATION USING ENCAPSULATED BONE
MORPHOGENETIC PROTEIN-2 IN POROUS POLYMER SCAFFOLDS
X. B. Yang1*, M. Watson2, W. Sebald3, S.
M. Howdle2, K. M. Shakesheff4, R. O. C. Oreffo1
1University Orthopaedics, University of Southampton, General
Hospital, Southampton, SO16 6YD, UK
2School of Chemistry, University of Nottingham, University
Park, Nottingham NG7 2RD, UK
3Department of Physiological Chemistry, University of
Wurzburg, Wurzburg, Germany
4School of Pharmaceutical Sciences, University of Nottingham,
University Park, Nottingham NG7 2RD, UK
The ability to deliver, over time, biologically active
osteogenic growth factors using designed scaffolds to sites of tissue regeneration offers
tremendous therapeutic implications in a variety of musculo-skeletal diseases. Thus the
encapsulation of osteogenic factors within biodegradable porous polymer scaffold may
provide an alternative approach to produce biomimetic osteogenic scaffolds for bone
regeneration. The aims of this study were to generate porous biodegradable scaffolds
encapsulating an osteogenic protein, bone morphogenetic protein-2 (BMP-2) and to examine
the ability of the scaffolds to promote human osteoprogenitor differentiation and bone
formation in vitro and in vivo.
BMP-2 encapsulated Poly(-lactic acid) (PLA) scaffolds
were generated using an innovative supercritical fluid process (Howdle et al 2001)*. After
encapsulation of rhBMP-2 within PLA scaffolds (100ng/mg PLA), bioactivity of rhBMP-2
encapsulated PLA scaffolds was confirmed by induction of the C2C12 promyoblast cell line
into the osteogenic lineage as detected by alkaline phosphatase expression. No induction
of alkaline phosphatase-positive cells was observed using blank scaffolds. BMP-2 released
from encapsulated constructs promoted adhesion, migration, expansion and differentiation
of human osteoprogenitor cells on 3-D scaffolds. Enhanced matrix synthesis and cell
differentiation on growth factor encapsulated scaffolds was observed following culture of
human osteoprogenitors for 7 days on explants of chick femoral bone wedge defects in an ex
vivo model of bone formation developed using the chick chorioallantoic membrane model. In
addition, BMP-2 encapsulated polymer scaffolds showed morphologic evidence of extensive
new bone and cartilage formation following subcutaneous implantation as well as within
diffusion chambers implanted into athymic mice as assessed by x-ray analysis,
immunocytochemistry with type I collagen antibody and alcian blue sirius red
histochemistry.
The generation of 3-D biomimetic structures incorporating
osteoinductive factors such as BMP-2 indicates their potential for de novo bone formation
that exploits cell- matrix interactions and, significantly, realistic delivery protocols
for growth factors in musculo-skeletal tissue engineering.
*Howdle et al Chem Comm. 2001, (1) 109-110
[Programme]
P-101
THE EFFECT OF LOW INTENSITY ULTRASOUND ON IN-VITRO OSTEOGENESIS
K. A. Hogan*, Y. H. An
Department of Orthopaedic Surgery, Medical University of South Carolina,
Charleston, SC, USA
Low intensity ultrasound (LIUS) has been shown to
accelerate fracture healing in animal and clinical studies. These studies have
standardized treatments to 20 minutes once a day but there is insufficient data to support
this choice of treatment duration. It is hypothesized that increasing treatment frequency
will increase osteoblast activity.
Bovine fetal osteoblasts were seeded at a density of 1.5
million cells per well and treated with LIUS for 28 days. The ultrasound unit
(Smith-Nephew) generated a pulsed (1.5 MHz) ultrasound wave (30-mW/cm2). Alkaline
phosphatase activity (ALP) was measured using a colorimetric assay (Sigma) and calcium
content was determined by ash weight.
Initially, cells were treated for 20 minutes a day with
LIUS; controls were not treated. ALP activity was measured every three days. Ash weight
was measured at days 21 and 28. Following verification of the model, a second group of
cells were treated with zero (control), one (1x), two (2x), or three (3x) LIUS treatments
per day. ALP activity was measured approximately every other day.
Mineralization was visible in both groups by day 14. LIUS
treated cells demonstrated earlier increases in ALP activity. ALP activity peaked in the
LIUS treated cells at day 15 compared to day 21 in the controls (p=0.002). Calcium content
was significantly increased in the LIUS group after 21 and 28 days of treatment (p=0.029).
These findings were replicated in the second experiment. Furthermore, ALP levels in the
LIUS 2x and 3x peaked significantly earlier (day 10 versus day 13) compared to the LIUS 1x
group (p=0.002) and reached higher peak values (p=0.004). There was a non-significant
trend towards an earlier peak in ALP expression in the LIUS 3x group compared to LIUS 2x.
In conclusion, LIUS positively affected mineralization in
osteoblasts as demonstrated by an earlier peak in alkaline phosphatase activity and an
increase in calcium content in the stimulated cells. Alkaline phosphatase activity
increased with more frequent treatments. These results indicate that increasing LIUS
treatments to two or three times per day may increase bone formation compared to one daily
20- minute treatment.
[Programme]
P-102
LONG BONE CRITICAL DEFECT IN RABBITS - TREATMENT WITH CANCELLOUS BONE,
BONE MARROW AND BMP-7
T. Djapic1, V. Kusec2*, M. Jelic1,3, M.
Pecina1, S. Vukicevic3
1Department of Orthopaedic Surgery, School of Medicine,
University of Zagreb, Croatia
2Clinical Institute of Laboratory Diagnosis, Clinical Hospital
Centre Zagreb, Croatia
3Department of Anatomy, School of Medicine, University of
Zagreb, Croatia
Compressed allogenic cancellous bone is an established
method for treatment of long bone defects. In order to accelerate bone healing autologous
bone marrow or 0.3 mg recombinant BMP-7 were combined with compressed allogenic cancellous
bone, or BMP-7 was used alone in the New Zealand rabbit experimental model of critical
ulnar defect (2 cm). Each experimental group comprised 6 adult male animals, including the
control group with no treatment of the defect and the group in which the defect was filled
with compressed cancellous bone only. All groups were monitored by x-ray during 10 weeks
when the experiment terminated. The outcome of the experiment in the control group and
group treated with compressed cancellous bone was non-union in all animals. Complete
healing of the bone defect in all experimental animals was achieved by a combination of
compressed cancellous bone and bone marrow. BMP-7 alone and in combination with compressed
cancellous bone induced healing in 50% of experimental animals. Histologic analysis of
bone specimens for groups treated with compressed cancellous bone and bone marrow or BMP-7
showed intensive bone turnover, small marrow spaces with fibrous tissue and abundant
capillaries. Healing with BMP-7 alone resulted with moderate bone turnover and larger
marrow spaces filled mostly with adipose tissue. In conclusion compressed cancellous bone
with either autologous bone marrow or BMP-7 promotes and supports healing of the critical
bone defect. BMP-7 alone produced most probably an early and time-limited stimulation of
bone formation, which was prolonged in combination with compressed cancellous bone.
Optimal retention of bone fragments was also essential for the outcome.
[Programme]
P-103
PROTEIN KINASE A ALTERS FLUID SHEAR INDUCED CA2+ SIGNALING IN OSTEOBLASTS
BY ALTERING ION CHANNEL KINETICS
R. L. Duncan*, J. Zhang, N. A. Ajubi, K. D. Ryder
Dept. of Orthopaedic Surgery, Indiana University School of Medicine,
Indianapolis, IN, USA
We have demonstrated that PTH increases the [Ca2+]i
response of osteoblasts to shear through enhanced activation of both mechanosensitive,
cation-selective channels (MSCC) and L-type voltage-sensitive Ca2+ (VSCC) channels. We
examined the role of PKA activation by these stimuli on ion channel activity and the
[Ca2+]i response in osteoblasts using the patch clamp and [Ca2+]i imaging. When MC3T3-E1
or UMR106.01 osteoblasts were subjected to fluid flow or PTH, the activity of a small, 5-6
pS channel increased from an NPo value (open time/total time for a single channel opening)
of 0.12 to 1.63. This increase was mimicked by addition of 8 br- cAMP and forskolin. This
channel conductance was the same as the increase in single channel conductance we had
previously reported for the MSCC in PTH-stimulated UMR106.01 cells, suggesting that,
rather than altering the MSCC channel kinetics, PTH-induced PKA activation was activating
a second channel. We also found that PKA activation reduced the peak activation of an
outwardly rectifying K+ channel. Addition of forskolin (0.01 mM) or 8 br-cAMP (0.1 mM)
reduced whole cell K+ currents by 40% within 10 min of treatment. To determine how the
interaction of these channels affected the [Ca2+]i response to shear, we sheared MC3T3-E1
cells at 12 dynes/cm2 in the presence of forskolin, 8 br-cAMP, or the PKA inhibitor H89
and measured the resultant [Ca2+]i response. PKA stimulation produced a peak [Ca2+]i
response to shear that was 87% greater than control responses. Additionally, the duration
of the [Ca2+]i response was increased by several minutes. When the outwardly rectifying K+
channel was inhibited with TEA and PKA was inhibited with H89, the peak [Ca2+]i response
to shear was reduced by 30%, but the duration of the [Ca2+]i response was similar to that
of cells treated with PKA agonists. These data would suggest that shear activates PKA to
increase the activity of a small conductance channel which enhances shear induced
depolarization to increase Ca2+ entry through L-type VSCC. The Ca2+ response is further
enhanced by reduced K+ currents to prevent rapid return of the membrane potential to
resting levels.
[Programme]
P-104
HYPOXIA AND ACIDOSIS DISRUPT BONE NODULE FORMATION BY RAT OSTEOBLASTS
I. R. Orriss*, A. Brandao-Burch, J. C. Utting, T. R. Arnett
Department of Anatomy & Developmental Biology, University College
London, London, UK
Reduced O2 levels cause impressive stimulation
of osteoclast generation in vitro, and acidosis, which is a consequence of tissue hypoxia,
appears to be the key requirement for activation of resorption pit formation. In this
study we investigated the effects of O2 and pH levels on osteoblast (OB)
function in vitro. OB were harvested from neonatal rat calvariae by digestion with trypsin
and collagenase and cultured for 5-7 d in DMEM / 10% FCS before plating at 104to
5x104/ well in 24-well plates with added ascorbate (0.05 mg/ml),
beta-glycerophosphate (2 mM) and 10 nM dexamethasone. For hypoxia experiments, plates were
cultured for up to 21d in sealed plastic boxes and gassed daily with 0.2 to 20% O2
(plus 5% CO2; balance N2). After fixation and staining with alizarin
red to demonstrate mineralisation, characteristic bone nodules, often with a 'trabecular'
morphology, were observed in 20 and 12% O2 cultures; however in 5, 2, 1 or 0.2%
O2, such nodules were absent and a single, amorphous mineralised mass was
present. In 0.2% O2 cultures, cell survival and/or proliferation was clearly
impaired. The operating pH of DMEM was typically 7.4 ±0.05, and was not significantly
altered by O2. For acidosis experiments, plates were cultured up to 21d in a
normal 5% CO2 incubator, and pH was reduced in some wells by addition of 5 to
25 mmol/l HCl. The effect of low pH (6.9 ±0.05) was to abolish completely the formation
of nodules, as well as any mineral deposition, the latter evidenced by alizarin red
staining, although cell growth appeared unimpaired. Even slight acidosis (pH ~7.3) caused
significant reduction of mineralised nodule formation. These experiments indicate that
bone formation may be critically dependent on O2(>~5%) and pH (>~7.3)
levels. Our findings may help explain why bone formation normally occurs close to blood
vessels in vivo and suggest that tissue hypoxia, with its attendant acidosis, is likely to
inhibit bone formation, in addition to stimulating osteoclastic resorption.
[Programme]
P-105
OP-1 (BMP-7) INDUCED HEALING OF A DISTAL TIBIAL NON- UNION IN THE PATIENT
PREVIOUSLY UNSUCCESSFULLY REOPERATED DURING 14 YEARS
M. Pecina1*, M. Haspl1, M. Jelic1,2, S.
Vukicevic2
1Department of Orthopedic Surgery, School of Medicine,
University of Zagreb, Zagreb, Croatia
2Department of Anatomy, School of Medicine, University of
Zagreb, Zagreb, Croatia
Following a distal tibial fracture due to falling, Z.I.
(male, then aged 49), was first unsuccessfully surgically treated by a plate and screws
which have been removed after 18 months. Next, he was unsuccessfully treated
conservatively by Sarmiento immobilization. One year later he was reoperated with a plate,
screws and autologous cancellous bone. Postoperatively, infection developed and
osteosynthetic material was removed. Next, a Thiersch graft was unsuccessfully applied due
to excessive skin damage. Following successful infection treatment, a gastrocnemius muscle
graft was applied. As the next procedure, non-union fragments were removed and Ilizarov
apparatus was applied, again unsuccessfully. Patient then refused amputation and orthosis
was applied in combination with two crutches which he used for walking until admittance to
our hospital. In April, 2001., the patient was admitted to the Department of Orthopedic
Surgery where fibula was disected and tibial non-union fragments were resected. Resected
fibula and some parts of tibia, that macroscopically appeared healthy, were grinded and
inserted around the tibial fragments. Two tibial ends were modeled as plates to make room
for the OP-1 device. Finally, the Ilizarov apparatus was applied. Soft tissues were
carefully sutured to secure the OP-1 device from dispersion. Tibial ends were then brought
closer by Ilizarov apparatus, but not compressed until 48 hours postoperatively. No
drainage was applied and skin was carefully sutured. After discharge, patient walked with
2 crutches and slowly advanced to full weight bearing during the next 12 months. X-rays
were taken every 2 months. The Ilizarov apparatus was removed 18 months postoperatively
after a complete radiographic healing on 4 cortices was observed and the patient continued
walking with one crutch for a month. After abandoning crutches, Z.I. walks with a 4 cm
higher shoe.
We conclude that OP-1 (BMP-7) device induced new bone
formation at the distal tibia following 14 years of unsuccessful multiple treatments of a
tibial non-union.
[Programme]
P-106
EXPRESSION OF BMP-3, BMP-7, CDMP-1 AND CDMP-2 IN HEALTHY AND
OSTEOARTHRITIC KNEE ARTICULAR CARTILAGE IN ELDERLY PEOPLE
D. Bobinac*, J. Spanjol, I. Maric, S. Zoricic
Department of Anatomy, School of Medicine, University of Rijeka, Croatia
Cartilage-derived morphogenetic proteins 1 and 2 (CDMP-1
and CDMP-2) are members of the bone morphogenetic protein (BMP) family. Localization
studies establish the involvement of CDMP-1 and CDMP-2 in the development, in the growth
and maintenance of cartilaginous tissues. Furthermore, BMP-7 is the only member of BMP
family, along with CDMPs, found to be expressed by chondrocytes in adult articular
cartilage. BMP-7 stimulates the synthesis of proteoglycans that may play a role in the
maintenance of a steady-state concentration of proteoglycans in human articular cartilage.
Some data show that the synthesis of proteoglycans in explants of bovine articular
cartilage is increased by BMP 3.
The early events in the OA are changes in the metabolism
and biochemistry of articular cartilage matrix. Great loss of cartilage tissue is a late
event. Thus increased degradation of cartilage matrix is a key event in the development of
OA. However, since cartilage matrix is continuously turned over under physiological
conditions, loss of matrix may be the result of defective mechanisms for replacement and
repair of matrix.
The aim was to investigate the expression of CDMPs and
BMPs in healthy articular cartilage from elderly people and OA cartilage. Ten cartilage
samples of knee joint were taken postmortem from human donors (70 yr) and from eleven
patients with knee joint osteoarthritis who underwent total knee arthroplasty. The samples
were stained with Safranin-O and by immunohistochemistry using polyclonal antibodies
specific for CDMP-1, CDMP-2, BMP-3, BMP-7. In the articular cartilage of elderly people
with strong reduction in proteoglycan staining we detected high level of CDMPs and BMPs
expression in the cells of all cartilage layers. In the tangential and partly in
transitional layers we found diffuses matrix positive staining or pericellular space
positive staining. In the articular cartilage without proteoglycan reduction, we found low
level of CDMPs and BMPs expression in the cells of transitional layer and matrix positive
staining in the superficial layer. In OA cartilage we found BMP-3 and BMP -7 cytoplasmatic
staining with very strong positive matrix staining. These results imply the role of CDMPs
and BMPs in maintenance of cartilage homeostasis, protection or repair.
[Programme]
P-107
TOWARDS THE GENERATION OF MONOCLONAL ANTIBODIES AGAINST PRIMITIVE MARROW
STROMAL CELLS BY PHAGE DISPLAY
J. Letchford1*, A. M. Cardwell1, K. Stewart2,
M. J. Perry3, J. N. Beresford1
1Bone Research Group, University of Bath, Bath, UK
2Dept. of Chemical Engineering, University of Bath, Bath, UK
3School of Veterinary Sciences, University of Bristol,
Bristol, UK
Marrow stroma contains rare clonogenic precursors (CFU-F)
which form colonies in vitro, and in vivo, differentiate into cells of different stromal
lineages. CFU-F and their progeny are not identifiable morphologically, and there are no
definitive stage and lineage specific markers. We are using phage display to generate
monoclonal antibodies against CFU-F enriched bone marrow mononuclear cells (BMMNC) to aid
the identification and isolation of CFU-F within human marrow stroma.
To identify an antibody best able to select for our
starting cell population, colony assays were performed to compare enrichment of CFU-F
following immuno-selection of different antibody defined sub-populations. BMMNC, isolated
by density gradient centrifugation of adult human marrow, were labelled with monoclonal
antibody and magnetic beads, then passed over a magnetic column. Positive and negative
fractions were plated at 20 000 cells/cm 2, fed twice weekly and after 14 -21
days, colony formation was assessed. When compared to unseparated BMMNC, selection of
CD49a positive cells produced the greatest (20 fold) enrichment of CFU-F; glycophorin A
negative CD45 negative BMMNC, and glycophorin A negative STRO- 1 positive BMMNC produced 0
and 9 and fold enrichment of CFU-F respectively.
We will use the highly diverse Griffin.1 phagemid library
to generate phage antibodies against CFU-F enriched BMMNC. Preliminary studies using MG-63
cells showed that 5 rounds of selection produced a 10 000 fold enrichment of library
phage, and highlighted the need for a simultaneous positive/negative selection procedure.
For this reason, library phage were incubated with unseparated BMMNC, and then bound phage
eluted from magnetically separated antibody-defined subpopulations. By optimising binding
and washing conditions, a mean phage titre of 2 x 105 colony forming units were
eluted from CD49a positive BMMNC after the first round of selection.
To summarise, we have identified anti CD49a as an
antibody that gives good enrichment of CFU-F, and by optimising phage selection
conditions, have obtained 2 x 105 clones from CD49a positive BMMNC after the
first round of selection. Production of phage antibodies against CFU-F enriched BMMNC, and
antibody screening is now in progress
[Programme]
P-108
MOLECULAR, CONFOCAL AND ULTRASTRUCTURAL STUDY ON RAT PY1A OSTEOBLASTS TO
ELUCIDATE FGF-2 AND FGFR2 BEHAVIOUR UNDER PROSTAGLANDIN TREATMENT
M. G. Sabbieti1*, L. Marchetti1, M. M. Hurley2,
G. Materazzi1, G. Menghi1
1Dept. Comparative Morphology and Biochemistry, University of
Camerino, Italy
2University of Connecticut Health Center, USA
We previously demonstrated, at molecular level, that
prostaglandin PGF2alfa and the synthetic analog fluprostenol (Flup) regulate
the expression of basic fibroblast growth factor (FGF-2) and the fibroblast growth factor
receptor 2 (FGFR2) mRNA via protein kinase C (PKC) mediated mechanism in rat Py1a
osteoblasts. Incubation with PD-98052 for 1 h and treatment with PGF2 alfa
demonstrated an involvement of MAPK kinase in this process. We found that sequential and
simultaneous double binding at confocal scanning laser microscopy (CLSM) contributes to
visualize and elucidate the spatial distribution of the two proteins FGF-2 and FGFR2
during accumulation and nuclear translocation in stimulated cells. These in situ findings
were also supported by parallel immunogold electron microscopy (IEM) studies.
Ultrastructural analysis demonstrated that an increment of coated-pits and coated-
vescicles occurs under PGF2 alfa treatment; however, double-sided binding
revealed that the cytoplasmic internalization of FGF-2 and its receptor 2 does not occur
at these structure level. A close correlation between FGF-2 and FGFR2 with importin beta
was also established at confocal and electron microscopy level.
[Programme]
P-109
AGONIST-DEPENDEMT CHANGES IN THE PHOSPHOLIPID COMPOSITION OF
OSTEOBLAST-LIKE MC3T3-E1 CELLS.
H. J. Leis*, W. Windischhofer, G. N. Rechberger, D. Zach, G. Fauler, H.
Köfeler
Univ. Childrens Hospital, University of Graz, Auenbruggerplatz 30, A-8036
Graz, Austria
Phospholipids serve as apool of esterified arachidonic
acid, the precursor of the biologically important prostaglandins. In bone, prostaglandin E2synthesised
by osteoblasts is a well known potent modulator of osteoblast and osteoclast function.
Thus, changes in the phospholipid composition may allow to trace for the main source
lipids of arachidonic acid after agonist stimulation. We have characterised the
phospholipid profile of MC3T3-E1 cells using liquid chromatography-mass spectrometry and
focussed on the changes of arachidonate-containing species after stimulation with various
agonists, like endothelin-1. The results show a significant dercrease of
arachidonate-containing phosphatidyl-inositol lipids as a consequence of phospholipase C
activation. The methodical approach offers various possibilities, such as isotope tracer
studies to follow arachidonate liberation and hence assign the liberation route to
different phospholipase pathways.
[Programme]
P-110
GENE EXPRESSION PROFILING OF HUMAN OSTEOBLAST-LIKE CELLS, HPOB-TERT,
DIFFERENTIATION AND MINERALIZATION
E. A. Federici, Y. Tromvoukis, A. Quintin, R. Mansourian, M. A. Roberts,
E. A. Offord*
Nestlé Research Center, CH-1000 Lausanne 26, Switzerland
Bone formation is a complex process that involves
osteoblast differentiation and mineralization and that is associated with specific changes
in gene expression. The number of genes known to be regulated during this process and
normally considered as 'osteoblast markers', is however still very limited. It is thus of
major interest to identify new genes that are activated or repressed during bone formation
as well as to better understand the interactions between genes already known to be
implicated in this differentiation pathway.
We have established a human osteoblast-like cell line,
hPOB-tert, immortalized by a combination of SV40 T-Ag and human telomerase gene
expression, as a bone formation model. Treatment of these cells with vitamin D3 and
dexamethasone (10- 8M) induced cell differentiation towards a mature osteoblast phenotype,
as monitored by an increase in alkaline phosphatase activity after 6 days and in vitro
mineralization demonstrated by calcium deposition after 21 days.
We examined the expression profiles of over 12000 genes
and ESTs represented in the Affymetrix human GeneChip during the in vitro differentiation
(day 2 and day 6 of treatment) and mineralization (day 14 and 21 of treatment) of
hPOB-tert cells. More than 7000 genes provided measurable signals. Many of the observed
changes, such as the induction of Cbfa1, alkaline phosphatase, osteocalcin and other
matrix remodeling factors, reflected expected patterns of gene expression and support the
physiological relevance of the results. A number of the observed changes involved genes
that have not been previously associated with osteoblast development. Understanding of the
role of these genes may offer new insights into the biological pathways regulated during
osteoblast commitment, differentiation and function, providing a more integrated
understanding of the bone formation process. Bioinformatic analysis such as gene
clustering and gene ontology annotation as well as validation of gene expression changes
by RealTime PCR are currently in progress and final results will be presented.
[Cell Biology: Osteoclasts and
Related Cytokines] |